��Ŀ����
(13��)�������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;��
��1���ڶ������У�������������������Ӧ�ų����������и�ֽ�÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߡ�
��2����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H��+468.2 kJ��mol-1
C(s)+O2(g)��CO2(g) ��H="-393.5" kJ��mol-1��
��Fe(s)��O2 (g)��Ӧ����Fe2 O3 (s)���Ȼ�ѧ����ʽΪ��_____________________��
��3������KMnO4��Һ�ζ�Fe2+��Ũ�ȣ���Ӧ�����ӷ���ʽ���£�5Fe2����MnO4����8H����5Fe3����Mn2����4H2O
��KMnO4��ҺӦʢ���ڣߣߣߣߣߵζ����У�
���жϴﵽ�ζ��յ�������ǣߣߣߣߣߣ�
���������ữ��0.020 00 mol��L-1��KMnO4��Һ�ζ�ijFeSO4��Һ���յ㣬ʵ�����ݼ�¼���±���
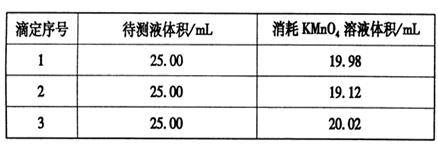
��������ݲ����㣬��FeSO4��Һ�����ʵ���Ũ��Ϊ�ߣߣߣߣߡ�
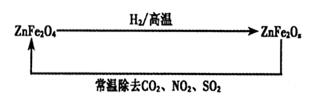
��4���������ײ���ZnFe2Ox�������ڳ�ȥ��ҵ�����е�ijЩ�������ȡ�²��Ϻͳ�ȥ������ת����ϵ����ͼ��
����֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1����ZnFe2Ox��x=�ߣߣߣߣߣ�
����ZnFe2Ox��ȥSO2�Ĺ����У��������ǣߣߣߣߣߡ�
��1���ڶ������У�������������������Ӧ�ų����������и�ֽ�÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߡ�
��2����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H��+468.2 kJ��mol-1
C(s)+O2(g)��CO2(g) ��H="-393.5" kJ��mol-1��
��Fe(s)��O2 (g)��Ӧ����Fe2 O3 (s)���Ȼ�ѧ����ʽΪ��_____________________��
��3������KMnO4��Һ�ζ�Fe2+��Ũ�ȣ���Ӧ�����ӷ���ʽ���£�5Fe2����MnO4����8H����5Fe3����Mn2����4H2O
��KMnO4��ҺӦʢ���ڣߣߣߣߣߵζ����У�
���жϴﵽ�ζ��յ�������ǣߣߣߣߣߣ�
���������ữ��0.020 00 mol��L-1��KMnO4��Һ�ζ�ijFeSO4��Һ���յ㣬ʵ�����ݼ�¼���±���

��������ݲ����㣬��FeSO4��Һ�����ʵ���Ũ��Ϊ�ߣߣߣߣߡ�
��4���������ײ���ZnFe2Ox�������ڳ�ȥ��ҵ�����е�ijЩ�������ȡ�²��Ϻͳ�ȥ������ת����ϵ����ͼ��

����֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1����ZnFe2Ox��x=�ߣߣߣߣߣ�
����ZnFe2Ox��ȥSO2�Ĺ����У��������ǣߣߣߣߣߡ�
��1��Fe2O3��2Al Al2O3��2Fe ��2��4Fe(s)+3O2 (g)��2Fe2O3(s) ��H����1648.7kJ/mol
Al2O3��2Fe ��2��4Fe(s)+3O2 (g)��2Fe2O3(s) ��H����1648.7kJ/mol
��3������ʽ ����Һǡ�ñ�dz�Ϻ�ɫ���Ұ�����ڲ���ɫ ��0.080 00 mol��L-1
��4����3.5 ��SO2
 Al2O3��2Fe ��2��4Fe(s)+3O2 (g)��2Fe2O3(s) ��H����1648.7kJ/mol
Al2O3��2Fe ��2��4Fe(s)+3O2 (g)��2Fe2O3(s) ��H����1648.7kJ/mol��3������ʽ ����Һǡ�ñ�dz�Ϻ�ɫ���Ұ�����ڲ���ɫ ��0.080 00 mol��L-1
��4����3.5 ��SO2
�����������1��������������Ӧ�����������ȷ�Ӧ������ʽΪFe2O3��2Al
 Al2O3��2Fe��
Al2O3��2Fe����2����֪����2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H��+468.2 kJ��mol-1����C(s)+O2(g)��CO2(g) ��H="-393.5" kJ��mol-1������ݸ�˹���ɿ�֪�ڡ�3���ټ��õ���Ӧ4Fe(s)+3O2 (g)��2Fe2O3(s)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����393.5 kJ/mol��3��468.2 kJ/mol����1648.7kJ/mol��
��3��������KMnO4��Һ����ǿ�����Ժ����ԣ�Ӧʢ������ʽ�ζ����У�
���������Ը��������Һ���Ϻ�ɫ�������жϴﵽ�ζ��յ����������Һǡ�ñ�dz�Ϻ�ɫ���Ұ�����ڲ���ɫ��
�۸��ݱ������ݿ�֪���ڶ���ʵ�������ĸ��������Һ�����������������ϴ���ȥ���ã�������ĸ��������Һ�����ƽ��ֵΪ
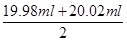 ��20.00ml�����Ը��ݷ���ʽ5Fe2����MnO4����8H����5Fe3����Mn2����4H2O��֪��FeSO4��Һ�����ʵ���Ũ��Ϊ
��20.00ml�����Ը��ݷ���ʽ5Fe2����MnO4����8H����5Fe3����Mn2����4H2O��֪��FeSO4��Һ�����ʵ���Ũ��Ϊ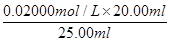 ��5��0.080 00 mol��L-1��
��5��0.080 00 mol��L-1����4������֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1�������ԭ���غ��֪����Ӧ�л�����1����ˮ������ZnFe2Ox��x��(4��2��1)��2��3.5��
����ZnFe2Ox����Ԫ�صĻ��ϼ���+2.5�ۣ�����Ӧ���Ϊ+3�ۣ����ϼ����ߣ�ʧȥ���ӣ�����ԭ��������������Ƕ�������

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ



 C��Ӧ�Ļ�ѧ����ʽ ��
C��Ӧ�Ļ�ѧ����ʽ �� +3Mn
+3Mn +24H+
+24H+ 5Fe3+ +10CO2��+3Mn2++12H2Oʵ�鷽�����Ϊ:
5Fe3+ +10CO2��+3Mn2++12H2Oʵ�鷽�����Ϊ:
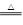 CeO2��8OH + 8_____����CeO2��8OH
CeO2��8OH + 8_____����CeO2��8OH
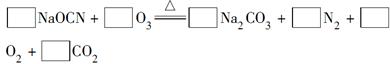
 �ṩ�����������й�������ȷ����( )
�ṩ�����������й�������ȷ����( ) 2 K2S + K2SO3 + 3 H2O
2 K2S + K2SO3 + 3 H2O