��Ŀ����
��һ�̶��ݻ����ܱ������У�����һ���¶ȣ���һ�������·������·�Ӧ��
2A(g)��B(g) 3C(g)��
3C(g)��
��֪����1 mol A��2 mol B�Ҵﵽƽ���������a mol C��
(1)�ﵽƽ��ʱ��C�ڷ�Ӧ������е����������______(�ú�a�Ĵ���ʽ��ʾ)��
(2)����ͬ��ʵ�������£�����ͬһ�����и�Ϊ����2 mol A ��4 mol B���ﵽƽ���C�����ʵ���Ϊ______mol(�ú�a�Ĵ���ʽ��ʾ)����ʱC�ڷ�Ӧ������е����������ԭƽ�����________(���������С�����䡱)��
(3)����ͬʵ�������£�����ͬһ�����и�Ϊ����2 mol A��5 mol B����Ҫ��ƽ���C�ڷ�Ӧ������е������������ԭƽ����ͬ����Ӧ����________ mol C��
2A(g)��B(g)
 3C(g)��
3C(g)����֪����1 mol A��2 mol B�Ҵﵽƽ���������a mol C��
(1)�ﵽƽ��ʱ��C�ڷ�Ӧ������е����������______(�ú�a�Ĵ���ʽ��ʾ)��
(2)����ͬ��ʵ�������£�����ͬһ�����и�Ϊ����2 mol A ��4 mol B���ﵽƽ���C�����ʵ���Ϊ______mol(�ú�a�Ĵ���ʽ��ʾ)����ʱC�ڷ�Ӧ������е����������ԭƽ�����________(���������С�����䡱)��
(3)����ͬʵ�������£�����ͬһ�����и�Ϊ����2 mol A��5 mol B����Ҫ��ƽ���C�ڷ�Ӧ������е������������ԭƽ����ͬ����Ӧ����________ mol C��
(1)a/3��(2)2a�����䡡(3)1
�����������1�����ݷ���ʽ��֪����Ӧǰ������Dz���ģ����Դﵽƽ��ʱ��C�ڷ�Ӧ������е����������
 ��
����2�����ڷ�Ӧǰ������Dz��䣬������ͬһ�����и�Ϊ����2 mol A ��4 mol Bʱƽ���ǵ�Ч�ģ����Դﵽƽ���C�����ʵ���Ϊ2amol����ʱC�ڷ�Ӧ������е����������ԭƽ����Ȳ��䡣
��3�����ڷ�Ӧǰ������Dz��䣬����Ҫ����ƽ���Ч���������Ͷ�ϱ�����ͬ�ġ���C�����ʵ���Ϊxmol����ת����A��B�����ʵ����ֱ�Ϊ2X/3��X/3�����У�2��2x/3��:��5��x/3����1:2�����x��1mol��
�������������е��Ѷȵ����⣬����ע�ػ��������������Ŀ����ѵ��������Ĺؼ�����ȷ��Чƽ����ж����ݣ�Ȼ��������������á������жϼ��ɣ�����������ѧ������˼ά�����ͳ���˼ά������

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
 2NH3�Ѵﵽƽ��״̬�� ��
2NH3�Ѵﵽƽ��״̬�� ��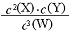
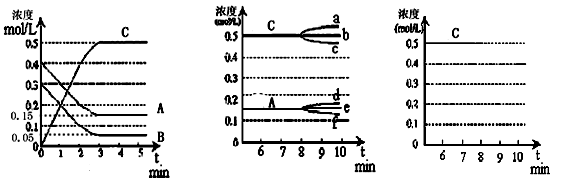
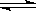 CO(g) + H2O(g) ��ƽ�ⳣ���˺��¶�t�Ĺ�ϵ���£�
CO(g) + H2O(g) ��ƽ�ⳣ���˺��¶�t�Ĺ�ϵ���£� ��ʱ�����ķ�Ӧ�������淴Ӧ���ʵĹ�ϵʽ�� ������ţ���
��ʱ�����ķ�Ӧ�������淴Ӧ���ʵĹ�ϵʽ�� ������ţ��� ��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����
��Ka��HSCN����0.13�����ܵ���ʵ��ܶȻ�������Kap��CaF2����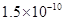
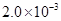 mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��
mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ��

 mol��L-1HF��Һ��
mol��L-1HF��Һ��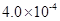 mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ�������������
mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯���� ����С����ޡ������������� b B��g����c C��g������ƽ������¶Ȳ��䣬�������������Ϊԭ����һ�������ﵽ�µ�ƽ��ʱ��c(A)��Ϊԭƽ���40%������˵����ȷ����
b B��g����c C��g������ƽ������¶Ȳ��䣬�������������Ϊԭ����һ�������ﵽ�µ�ƽ��ʱ��c(A)��Ϊԭƽ���40%������˵����ȷ���� H2O(g) + CO(g)����Ӧ�������¶���ͬ������ʼŨ�Ȳ�ͬ������ �ף�n(CO2)��n(H2)��1 mol;�ң�n(CO2)��1 mol, n(H2)��2mol������n(CO2)��n(H2)��1mol n[H2O(g)]��1 mol.�ﵽƽ��ʱCO�����ʵ����ɴ�С��˳����( )
H2O(g) + CO(g)����Ӧ�������¶���ͬ������ʼŨ�Ȳ�ͬ������ �ף�n(CO2)��n(H2)��1 mol;�ң�n(CO2)��1 mol, n(H2)��2mol������n(CO2)��n(H2)��1mol n[H2O(g)]��1 mol.�ﵽƽ��ʱCO�����ʵ����ɴ�С��˳����( ) 2SO3( g ) �� �� H =��QkJ��mol-1
2SO3( g ) �� �� H =��QkJ��mol-1