��Ŀ����
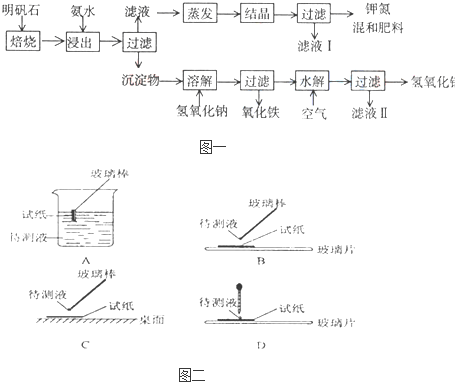
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��
��������ͼʾ�����������գ�
������ʯ���պ���ϡ��ˮ����������500 mLϡ��ˮ(ÿ������39.20 g��)��ҪȡŨ��ˮ��ÿ������251.28 g�����ߣߣߣߣߣ�mL���ù��Ϊ�ߣߣߣߣߣ�mL��Ͳ��ȡ��
�ư�ˮ������õ���Һ�����ϵ�����ˣ���Һ�г�K����SO42���⣬���д�����NH4����
����NH4���ķ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ� �ߣߡ�
��д�����������������ʵĻ�ѧʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
����ҺI�ijɷ���ˮ�ͣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ������������в��裺
�ٳ�ȡ�ص�������������ˮ�����������ߣߣߣߣߣߣߣ���Һ��������ɫ������
�ڣߣߣߣ� ���ߣߡ��ߣߣߣߣߣߣߡ��ߣߣߣߣߣߣߣ�������дʵ��������ƣ���
����ȴ�����ء�
��������Ϊm g�����������ʵ���Ϊ n mol����������K2SO4�����ʵ���Ϊ���� ���ߣ� mol(�ú�m��n�Ĵ���ʽ��ʾ)��
��1��78.00��100
��2��ȡ��Һ�������Թ��У�����ŨNaOH��Һ�����ȣ����ɵ�������ʹʪ��ĺ�ɫʯ����ֽ����������
��3��Al(OH)3��Al2O3��Fe2O3
��4��K2SO4��(NH4)2SO4
��5����BaCl2��Ba(OH)2 �ڹ��ˡ�ϴ�ӡ�������ɣ�
��6��mol

 ��������������������ϵ�д�
��������������������ϵ�д�