��Ŀ����
����Ŀ��I�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
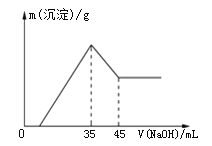
��1�������a������_______________��
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������______������ͼ����ĸf��g����
��3�������ø�װ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵIJ���������______��
��4�����ø�װ��������ˮ��ʵ��ʱb�г�������������ˮ�⣬��������������Ƭ����������_________________��
II����98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3������100mL 1.0mol��L-1ϡ���ᣬʵ�鲽�����£��ټ�������Ũ������������ȡһ�������Ũ�����ϡ�ܼ͢�©��ת�ơ�ϴ�Ӣݶ��ݡ�ҡ�ȡ���ʵ�������У�A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ E��10mL��Ͳ F����ͷ�ι� G��50mL�ձ� H��100mL����ƿ���ش��������⣺
��1������ȡŨ��������Ϊ___________mL��
��2��ʵ��ʱѡ�õ�������___________������ţ���
��3�����ƹ����У����������ʹ���ƽ��ƫ�ߵ���___________������ţ���
�ٶ���ʱ���ӿ̶���
������ƿʹ��ʱδ���������������ˮ
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
������Ͳ��ȡŨ����ʱ���Ӷ���
�������ƵĹ����У�����ȡ�õ�Ũ���ᵹ���ձ���ϡ�ͣ��ձ��е���Һδ����ȴ����ת��������ƿ�У��漴����
���𰸡������ܣ���ֱ�������ܣ� g �¶ȼ� ��ֹ���� 5.4 CEFGH �٢ܢ�
��������
I��(1)����aΪֱ�������ܣ�
(2)����ˮ��ˮ������Ϊ�½��ϳ���
(3)�����¶ȼƿ����¶ȣ�
(4)���Ƭ�ɷ�ֹҺ����ҷ��ڣ�
II��(1)��Һϡ��ʱ�����ʵ����ʵ������䣻
(2)����ʵ�鲽��ѡ�õ�������E��10mL��Ͳ��G��50mL�ձ���C����������H��100mL����ƿ��F����ͷ�ιܣ�
(3)�ٶ���ʱ���ӿ̶��ߣ�������Һ�����ƫС��
������ƿʹ��ʱδ���������������ˮ����Һ�������Ӱ�죬���ʵ�������Ӱ�죻
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߣ�������Һ�����ƫ��
������Ͳ��ȡŨ����ʱ���Ӷ�����������ȡŨ��������ƫ�����ʵ�����ƫ��
�������ƵĹ����У�����ȡ�õ�Ũ���ᵹ���ձ���ϡ�ͣ��ձ��е���Һδ����ȴ����ת��������ƿ�У��漴���ݣ�����������Һ�����ƫС��
I��(1)����aΪֱ�������ܣ�
(2)����ˮ��ˮ������Ϊ�½��ϳ�����f����
(3)���÷е㲻ͬ���з��룬ȱ���¶ȼƣ�
(4)���Ƭ�ɷ�ֹҺ����ҷ��ڣ�
II��(1)��Һϡ��ʱ�����ʵ����ʵ������䣻Ũ�����Ũ��c=![]() =
=![]() =18.4mol/L��18.4mol/L��V=1.0mol��L-1��100mL��V=5.4mL��
=18.4mol/L��18.4mol/L��V=1.0mol��L-1��100mL��V=5.4mL��
(2)����ʵ�鲽��ѡ�õ�������E��10mL��Ͳ��G��50mL�ձ���C����������H��100mL����ƿ��F����ͷ�ιܣ�
(3)�ٶ���ʱ���ӿ̶��ߣ�������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
������ƿʹ��ʱδ���������������ˮ����Һ�������Ӱ�죬���ʵ�������Ӱ�죬������Һ��Ũ�Ȳ��䣻
�۶��ݺ���ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߣ�������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
������Ͳ��ȡŨ����ʱ���Ӷ�����������ȡŨ��������ƫ�����ʵ�����ƫ��������Һ��Ũ��ƫ�ߣ�
�������ƵĹ����У�����ȡ�õ�Ũ���ᵹ���ձ���ϡ�ͣ��ձ��е���Һδ����ȴ����ת��������ƿ�У��漴���ݣ�����������Һ�����ƫС��Ũ��ƫ�ߣ�
������������Ϊ�٢ܢݡ�

����Ŀ��ij��ѧ��Ӧ�У���Ӧ�����A��B��C�����ʵ���Ũ��(c)��ʱ��(t)��ϵ���±���ʾ��
��ʼ | 2 min | 4 min | 6 min | |
c (A)(mol/L) | 1.45 | 1.28 | 1.00 | 1.00 |
c (B)(mol/L) | 0.38 | 0.72 | 1.28 | 1.28 |
c (C)(mol/L) | 0.095 | 0.18 | 0.32 | 0.32 |
����˵����ȷ���ǣ� ��
A.�÷�Ӧ�Ļ�ѧ����ʽΪA = 2B+C
B.4 minĩA��ת����ԼΪ31%
C.4~6minʱ����Ӧֹͣ��
D.����Ӧ�����ȷ�Ӧ