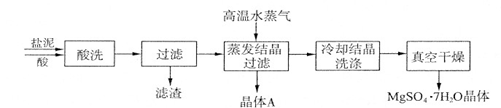
��Ŀ����
ʵ�����Ʊ����ᶡ������Ӧ�¶�Ҫ������115��~125��֮�䣬�����й��������±���
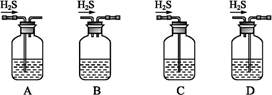
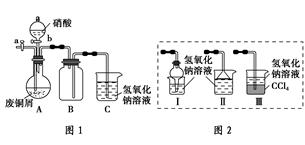
����ʵ�����Ʊ����ᶡ���������������
A�����ܱ߷�Ӧ���������ᶡ����ԭ�����ᶡ���ķе��
B������ˮԡ��������Ϊ�����ᶡ���ķе����100��
C���ӷ�Ӧ������������Ʒ�ķ�������Na2CO3��Һϴ�Ӻ��Һ
D���ɴ�Ʒ�ƾ�Ʒ��Ҫ���е�һ������������ˮ������
| ���� | ���� | 1?���� | ���ᶡ�� | 98%Ũ���� |
| �е� | 117.9�� | 117.2�� | 126.3�� | 338.0�� |
| �ܽ��� | ����ˮ���л��ܼ� | ����ˮ���л��ܼ� | ����ˮ�������л��ܼ� | ��ˮ���� |
����ʵ�����Ʊ����ᶡ���������������
A�����ܱ߷�Ӧ���������ᶡ����ԭ�����ᶡ���ķе��
B������ˮԡ��������Ϊ�����ᶡ���ķе����100��
C���ӷ�Ӧ������������Ʒ�ķ�������Na2CO3��Һϴ�Ӻ��Һ
D���ɴ�Ʒ�ƾ�Ʒ��Ҫ���е�һ������������ˮ������
B
����������ɱ������ݿ��Կ������ᶡ���ķе�Ƚϸߣ���˲��ܱ߷�Ӧ���������ᶡ����ԭ�����ᶡ���ķе�ߣ���ȷ��B����Ӧ�¶�Ҫ������115��~125��֮�䣬ˮԡ�����¶�ֻ�ܴﵽ100�棬����C����Na2CO3��Һ�ɳ�ȥ����Ȼ��ϴ�Ӻ��Һ����ȷ��D����ˮ���������������ȷ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


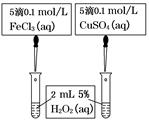
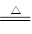 ________Cu��________CO2����________H2O
________Cu��________CO2����________H2O FeSO4+H2��
FeSO4+H2��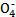 +5C2
+5C2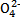 +16H+
+16H+