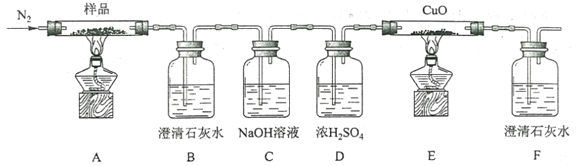
��Ŀ����
����Ŀ��(1)������PbSO4����CH3COONH4��Һ���Ƶ�������ˮ��(CH3COO)2Pb�������ķ�ӦΪPbSO4��2CH3COONH4��(CH3COO)2Pb��(NH4)2SO4��˵��(CH3COO)2Pb��________(����ǿ����������)����ʡ�
(2)��֪������(H3PO2)������������������Һ��Ӧ����NaH2PO2��H2O�����������_____Ԫ��(����һ������������������)��
(3)��ҵ���Ʋ�����ˮ�����õ��Ĺ�ͬԭ����________(�ѧʽ)��
(4)��һ���¶��£���һ��2 L������ܱ�������(Ԥ��װ�����)ͨ��1 mol N2��3 mol H2��������Ӧ��N2(g)��3H2(g)2NH3(g)������һ��ʱ����������ѹǿ����ʼ��0.9�����ڴ�ʱ���ڣ�H2ƽ����Ӧ����Ϊ0.1 mol/(L��min)������������ʱ��Ϊ______min
(5)��������(Na2FeO4)����ǿ�����ԣ��ɶ�����ˮ�����������������������ƿ������������ʹ��������ڼ��Խ����з�Ӧ�õ����벹�䲢��ƽ�������ӷ���ʽ��
____Fe(OH)3 +____ClO��+____OH��=__FeO42����___Cl��+__
(6)�ڷ�Ӧ11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4�У���������___________������2mol H3PO4���ɣ�ת�Ƶĵ��ӵ����ʵ���Ϊ___________
���𰸡��� һ CaCO3 3 2 3 4 2 3 5H2O P��CuSO4 10mol
��������
(1)����Ǧ��ˮ��Һ������ܽ�ƽ�⣬��������ʱ����������Ӻ�Ǧ���������ѵ������ʻ�������ʴ���Ǧ����ʹ����Ǧ�ܽ⣬˵��(CH3COO)2Pb��������ʣ�
(2)H3PO2��������NaOH��Һ��Ӧ������NaH2PO2��˵��H3PO2ֻ�ܵ����һ�������ӣ�����H3PO2��һԪ�
(3)ˮ�������ԭ��Ϊ�����ʯ��ʯ���Ʋ�������Ҫԭ��Ϊ���ʯ��ʯ��ʯӢɰ����ͬԭ��ΪCaCO3��
(4)�������Ϊ2L�����Գ�ʼͶ��c(N2)=0.5mol/L��c(H2)=1.5mol/L�����c(N2)=x mol��������ʽ�У�

������ѹǿ����ʼ��0.9�����¶Ⱥ������ݻ����䣬�����ѹǿ�ȵ���Ũ��֮�ȣ�����![]() �����x=0.1mol/L�����c(H2)=0.3mol/L��v(N2)=0.1 mol/(L��min)����Ӧʱ��t=
�����x=0.1mol/L�����c(H2)=0.3mol/L��v(N2)=0.1 mol/(L��min)����Ӧʱ��t=![]() =3min��
=3min��
(5)��Ԫ�ػ��ϼ���+3����Ϊ+6��ʧȥ3�����ӣ���Ԫ�ػ��ϼ���+1����Ϊ-1����2�����ӣ�ȡ��С��������������������������ǰϵ��Ϊ2�����������������ǰϵ��Ϊ3���ٸ��ݵ���غ㼰ԭ���غ���ƽ��2Fe(OH)3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��
(6)Cu3P��PԪ�ػ��ϼ�Ϊ-3��H3PO4��PԪ�ػ��ϼ�Ϊ+5������P�������������ǻ�ԭ����CuԪ�ػ��ϼ���CuSO4�е�+2����ΪCu3P��+1������CuSO4Ҳ������������������ΪP��CuSO4������2molH3PO4���ɣ�ת�Ƶĵ��ӵ����ʵ���Ϊ2mol��(+5-0)=10mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�