��Ŀ����
����Ŀ��̼���仯����㷺��������Ȼ���С��ش��������⣺
(1)��̬̼ԭ�Ӻ�����________��������ͬ�ĵ��ӣ� ���������˶�״̬��_____ �����ؾ�����
(2)CH4���Ӽ䲻���γ������ ��Ҫԭ����CH4 �����е�̼ԭ�Ӳ����¶Ե��ӡ�_____ �� _____________��
(3)̼��ķ��ǻ����ĸ���������ķ��ǻ����ĸ�������1, �ӽṹ�Ϸ��������ǵ�ǿ�� �������Ϊ��ǿ�ᡣȻ����ʵ�϶�����̼ˮ��Һ������ȴ������ԭ����__________��
(4)�Ҷ���(H2NCH2CH2NH2)�� һ���л������ N ԭ�ӵ��ӻ��������Ϊ______���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ���ṹ�ɱ�ʾΪ__________��
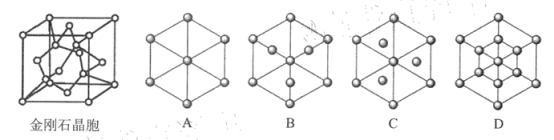
(5)���ʯ��̼��һ��ͬ�������壬����_______ ���塣��֪�����Ƶľ��������������ѻ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ��ͼA ��ʾ������ʯ����������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦ������ͼ ___________���� A~D ͼ��ѡ���
��̼ԭ�Ӱ뾶Ϊr ,���ʯ������̼ԭ�ӵĿռ�ռ����Ϊ_____________�� �ú��� �Ĵ���.ʽ��ʾ����
���𰸡�3 4 �縺�Խ�С ԭ�Ӱ뾶�ϴ� ����ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�ᣨ�������ɣ� sp3 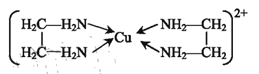 ԭ�� D
ԭ�� D ![]()
��������
(1)ͬһ�ܼ��еĵ���������ͬ����ͬ�ܼ��е���������ͬ��
(2)����������γ����������ʷ������
(3)������ķ��ӽṹ��������̼��ˮ�е��ܽ�ȷ������
(4) ���ݼ۲���ӶԻ���ģ�ͷ�����𣻸�����λ�����γ�ԭ��������λ���ӵĽṹ��
(5)�������ʵ��۷е㡢Ӳ�ȵ����������жϾ������ͣ����ݾ����Ŀռ�ṹ�������
(1)̼ԭ�Ӻ�����6�����ӣ���������Ų�ʽΪ1s22s22p2����1s��2s��2p 3���ܼ����ʺ�����3��������ͬ�ĵ��ӣ����������˶�״̬��4�����ؾ������ֱ�Ϊ��a.���Ӳ㣨��������n����b.�����Dz�͵����Ƶ���״�������������������l����c.�����Ƶ���չ����������m����d.���ӵ�����������������ms����
�ʴ�Ϊ��3��4��
(2)�γ�����������У�1.������縺�Ժܴ��ԭ��A�γ�ǿ���Լ�����ԭ�ӣ�2.���ڽ�С�뾶���ϴ�縺�ԡ����¶Ե��ӡ����в��ָ���ɵ�ԭ��B (F��O��N)��CH4���Ӽ䲻���γ������ ��Ҫԭ����CH4 �����е�̼ԭ�Ӳ����¶Ե��ӡ��縺�Խ�С��ԭ�Ӱ뾶�ϴ�
�ʴ�Ϊ���縺�Խ�С��ԭ�Ӱ뾶�ϴ�
(3)������̼ˮ��Һ������ȴ������ԭ��������ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�
�ʴ�Ϊ������ˮ�Ķ�����̼����ֻ�в�����ˮ��ϳ�̼�ᣨ�������ɣ���
(4)�Ҷ�����Nԭ�ӵ��ӻ����������NH3��ͬ�����ݼ۲���ӶԻ���ģ�ͷ���������ԭ��N�ļ۲���Ӷ���Ϊ![]() �������ӻ�����Ϊsp3���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ�Cu2+����λ��Ϊ4������ṹ�ɱ�ʾΪ
�������ӻ�����Ϊsp3���Ҷ���ͨ����λ������Cu2+ �γ��ȶ��Ļ�״�����ӣ�Cu2+����λ��Ϊ4������ṹ�ɱ�ʾΪ ��
��
�ʴ�Ϊ��sp3�� ��
��
(5)���ʯ�۵�ߡ�Ӳ�ȴ�����ԭ�Ӿ��壻���ʯ�ṹ���Ƽ���ռ乹�͵���������ṹ��������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦΪ������������Σ�����侧��ͼ��֪Ӧ��ͼD�����ʯ������ͼ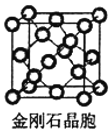 ���þ�����Cԭ�Ӹ���4+8��
���þ�����Cԭ�Ӹ���4+8��![]() +6��
+6��![]() =8�����ʯ��Խ����ϵ��ĸ�ԭ�ӽ��������������ⳤa=
=8�����ʯ��Խ����ϵ��ĸ�ԭ�ӽ��������������ⳤa=![]() ���������=a3=
���������=a3=![]() ������ԭ�����=
������ԭ�����=![]() ���ռ�ռ����=
���ռ�ռ����=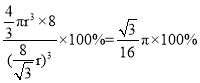 ��
��
�ʴ�Ϊ��ԭ�ӣ�D��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ѧ������������ء�
I.K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72-(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ��
��1����̬Crԭ�ӵļ۵��ӹ������ʽΪ__��
��2��CH3COOH����������Ԫ�صĵ縺���ɴ�С��˳��Ϊ__��̼ԭ�ӵĹ���ӻ�����Ϊ__��������������������Ŀ֮��Ϊ__��
��3����֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ�����ʾ��
���� | Sc3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ__��
II.ZnCl2Ũ��Һ�����ڳ�ȥ��������������������FeO��Ӧ�ɵ�Fe[Zn(OH)Cl2]2��Һ��
��4��Fe[Zn(OH)Cl2]2��Һ�в����ڵ�������������__(��ѡ����ĸ)��
A.���Ӽ� B.���ۼ� C.������ D.��λ�� E.���»��� F.���
��Һ��[Zn(OH)Cl2]-�ĽṹʽΪ__��
III.п������������Ԫ��֮һ����ѻ���ʽ��ͼ1�������ṹ��ͼ2��
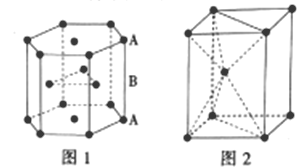
��5��п�Ķѻ���ʽΪ__����λ��Ϊ__��
��6����пԭ�ӵİ뾶Ϊapm�������ӵ�������ֵΪNA����п������ܶ�Ϊ___g/cm3(�ú�a�Ĵ���ʽ��ʾ)��