��Ŀ����
ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
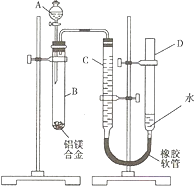
��1��A���Լ�Ϊ______��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ�����н���Ƭ�̣���Ŀ����______��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԣ�����������˳����______������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ______��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��5����ʵ������þ�Ͻ������Ϊag������������ΪbmL���ѻ���Ϊ��״������B��ʣ���������Ϊcg�����������ԭ������Ϊ______��
��6��ʵ������У���δϴ�ӹ������õIJ����������������������______���ƫ����ƫС����������Ӱ�족����
��1��A���Լ�Ϊ______��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ�����н���Ƭ�̣���Ŀ����______��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԣ�����������˳����______������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ______��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��5����ʵ������þ�Ͻ������Ϊag������������ΪbmL���ѻ���Ϊ��״������B��ʣ���������Ϊcg�����������ԭ������Ϊ______��
��6��ʵ������У���δϴ�ӹ������õIJ����������������������______���ƫ����ƫС����������Ӱ�족����

��1��������þ�Ļ�ѧ���ʣ���þ�������ᷴӦ�ų�������������������NaOH��Һ����Ӧ�ų���������þ���ܣ�Ҫ�ⶨ��þ�Ͻ�����������������Ӧѡ��NaOH��Һ��
�ʴ�Ϊ��NaOH��Һ��
��2����þ�ı��涼�����γ�һ������Ĥ����ʵ��ǰ�����ȥ���ʴ�Ϊ����ȥ��þ�Ͻ���������Ĥ��
��3��ʵ��ʱ����Ҫ��������ԣ�������������C��Һ��λ�ã��ټ���NaOH��Һ��ʼ��Ӧ������Ӧ��ϲ���ȴ�����º�¼��������C��Һ��λ�ã����B��ʣ�������ˣ�ϴ�ӣ�������أ������ܶ���ʱΪʹ��������ѹǿ��������ѹ��ȣ�����ʹD��C������Һ����ƽ��
�ʴ�Ϊ���ݢ٢ܢۢڣ�ʹD��C��Һ����ƽ��
��4��B���з�������NaOH��Һ�ķ�Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��5����þ�Ͻ������Ϊa g��B��ʣ�����þ������Ϊc g����μӷ�Ӧ����������Ϊ��a-c��g�����������ԭ������ΪM����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
2M3��22400ml
��a-c��gbml
��֮�ã�M=
���ʴ�Ϊ��
��
��6����������������
��100%��ʵ������У���δϴ�ӹ������õIJ����cֵƫ��������������ƫС��
�ʴ�Ϊ��ƫС��
�ʴ�Ϊ��NaOH��Һ��
��2����þ�ı��涼�����γ�һ������Ĥ����ʵ��ǰ�����ȥ���ʴ�Ϊ����ȥ��þ�Ͻ���������Ĥ��
��3��ʵ��ʱ����Ҫ��������ԣ�������������C��Һ��λ�ã��ټ���NaOH��Һ��ʼ��Ӧ������Ӧ��ϲ���ȴ�����º�¼��������C��Һ��λ�ã����B��ʣ�������ˣ�ϴ�ӣ�������أ������ܶ���ʱΪʹ��������ѹǿ��������ѹ��ȣ�����ʹD��C������Һ����ƽ��
�ʴ�Ϊ���ݢ٢ܢۢڣ�ʹD��C��Һ����ƽ��
��4��B���з�������NaOH��Һ�ķ�Ӧ��2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��5����þ�Ͻ������Ϊa g��B��ʣ�����þ������Ϊc g����μӷ�Ӧ����������Ϊ��a-c��g�����������ԭ������ΪM����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
2M3��22400ml
��a-c��gbml
��֮�ã�M=
| 33600(a-c) |
| b |
| 33600(a-c) |
| b |
��6����������������
| (a-c) |
| a |
�ʴ�Ϊ��ƫС��

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ