��Ŀ����
����Ŀ����Ԫ�ؿ��γɶ��ֻ�����ڹ�ҵ�����о�����Ҫ��ֵ�� ��ش��������⣺
��1����֪��1mol H��H ����1mol N��H���� 1mol N��N���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ���Ҹ÷�ӦΪ���淴Ӧ����N2��H2��Ӧ�ϳ�NH3���Ȼ�ѧ����ʽΪ_________��
��2��һ���¶��£���һ������N2��H2����̶�������ܱ������н��кϳɰ���Ӧ��
������������˵���ÿ��淴Ӧ�ﵽ��ѧƽ��״̬����___________
A.������������ܶȲ��� B.c(N2)��c(H2)��c(NH3)=1��3��2
C.�����ڵ�ѹǿ���� D.3v��(H2) =2v��(NH3)
E. �����������ƽ����Է�����������
F. ��ͬʱ������3molH-H�����ѣ���6mol N-H���γ�
�ں��º�ѹ�����£�Ϊ��ߺϳɰ���Ӧ��N2��H2�������ʣ����Բ��õķ�����________________��
��3��һ���¶��£�2L�ܱ������г���0.40 mol N2O4��������Ӧ��N2O4(g)![]() 2NO2(g)��һ��ʱ���ﵽƽ�⣬����������£�
2NO2(g)��һ��ʱ���ﵽƽ�⣬����������£�
ʱ�䣯s | 20 | 40 | 60 | 80 | 100 |
c(NO2)/(mol/L) | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
��20s�ڣ�v(NO2)=___________�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ_________��
�������¶�ʱ��������ɫ���������Ӧ��_________(��������������������)��Ӧ��
����ͬ�¶��£�����ʼ��������г���0.40 mol NO2����ﵽƽ���: c(NO2)_____0.15 mol��L-1(����>���� ��=������<��)
���𰸡�N2(g)+3H2(g)![]() 2NH3(g) ��H��-92 kJ��mol��1CE��ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ������0.006 mol��L-1��s-11.8���ȣ�
2NH3(g) ��H��-92 kJ��mol��1CE��ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ������0.006 mol��L-1��s-11.8���ȣ�
��������
(1)�ڷ�ӦN2+3H22NH3�У�����3molH-H����1molN��N�������յ�����Ϊ��3��436kJ+946kJ=2254kJ������2molNH3ʱ���γ�6molN-H�����ų�������Ϊ��6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������=2346kJ-2254kJ=92kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)2NH3(g)��H=-92kJmol-1���ʴ�Ϊ��N2(g)+3H2(g)2NH3(g)��H=-92kJmol-1��
(2)��A���÷�Ӧ�л�����������������ݻ�Ϊ��ֵ������������ܶ�ʼ�ղ��䣬���ܸ��ݻ��������ܶ��ж�ƽ��״̬����A����B��c(N2)��c(H2)��c(NH3)=1��3��2�����жϸ���ֵ�Ũ���Ƿ�����仯�������ж�ƽ��״̬����B����C���÷�ӦΪ���������С�ķ�Ӧ��ѹǿΪ�������������ڵ�ѹǿ���䣬�������淴Ӧ������ȣ��÷�Ӧ�ﵽƽ��״̬����C��ȷ��D.��Ӧ����ƽ��ʱ��2v��(H2)=3v��(NH3)����D����E. �÷�Ӧ����������ʵ�����С�ķ�Ӧ����������ʵ���Ϊ����������![]() =
=![]() �������������ƽ����Է����������䣬˵������������ʵ������䣬�ܹ�˵���ﵽƽ��״̬����E��ȷ��F����ͬʱ������ 3molH-H �����ѣ��� 6molN-H ���γɣ���ʾ�Ķ�������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���F���ʴ�Ϊ��C E��
�������������ƽ����Է����������䣬˵������������ʵ������䣬�ܹ�˵���ﵽƽ��״̬����E��ȷ��F����ͬʱ������ 3molH-H �����ѣ��� 6molN-H ���γɣ���ʾ�Ķ�������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���F���ʴ�Ϊ��C E��
�� ���º�ѹ�����£�Ϊ��ߺϳɰ���Ӧ��N2��H2�������ʣ���ƽ����������Ӧ�����ƶ������Բ��õķ����м�ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ�����õȣ��ʴ�Ϊ����ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ�����ã�
(3)��20sʱNO2��Ũ��Ϊ0.12mol/L����20s���ö���������ʾ��ƽ����Ӧ����v(NO2)=![]() =0.006molL-1s-1����ʼʱc(N2O4)=
=0.006molL-1s-1����ʼʱc(N2O4)=![]() =0.2mol/L�����ݱ������ݣ�ƽ��ʱc(NO2)=0.3mol/L����c(N2O4)=0.2mol/L-0.15mol/L=0.05mol/L����ѧƽ�ⳣ��K=
=0.2mol/L�����ݱ������ݣ�ƽ��ʱc(NO2)=0.3mol/L����c(N2O4)=0.2mol/L-0.15mol/L=0.05mol/L����ѧƽ�ⳣ��K=![]() =1.8���ʴ�Ϊ��0.006 molL-1s-1��1.8��
=1.8���ʴ�Ϊ��0.006 molL-1s-1��1.8��
�������¶�ʱ��������ɫ���˵�����º�ƽ�������ƶ���������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
����ͬ�¶��£�����ʼ��������г���0.40 mol NO2���൱�ڿ�ʼ��������г���0.20 mol N2O4����������������䣬���൱�ڼ�Сѹǿ��ƽ�������ƶ��������ﵽƽ���: c(NO2)����ԭ����һ�룬��c(NO2)��0.15 mol��L-1���ʴ�Ϊ������

����Ŀ�����ǵ�IIIA��Ԫ�أ��������ڼ���������������ַǽ�����Ӧ��ijͬѧ��������������������Ӧ�Ʊ����Ȼ�����֪BC13�ķе�Ϊ12.5 �� ���۵�Ϊ-107.3 �棬��ˮ���ҷ�Ӧ��������������ᡣ��ͬѧѡ����ͼ��ʾ�IJ���װ�ã������ظ�ѡ�ã�����ʵ�飬��ش��������⣺
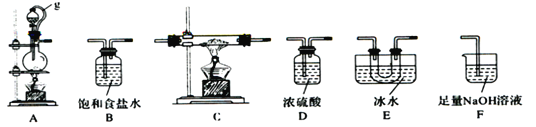
��1��A�з�Ӧ�����ӷ���ʽΪ__________________��
��2��ͼ��g�ܵ�������______________________________________��
��3��װ�õ�����˳������ΪA�� �� �� ��E��D��F��____________��E��Fװ�ü�����Dװ�õ�������____________________________________________________________��
��4��ֹͣʵ��ʱ����ȷ��ʵ�������______________________________________________________________________________________________________________��
��5��������(H3BO3)ΪһԪ���ᣬ��������NaH2BO3Ϊ_____������Ρ�����ʽ�Ρ���ʽ�Ρ�����
��6��ʵ����ɺ�ijͬѧ��F�У���Һ����0.05mol/LNaC1O�� 0.05mol/LNaCl��0.1mol/LNa0H���μ�Ʒ����Һ��������Һ��ɫ�������ʵ��̽����Һ��ɫ��ԭ�����ڱ��пո��������ݣ����ʵ�鷽����
ʵ����� | 0.1mol/LNaClO��Һ/mL | 0.1mol/LNaCl��Һ/mL | 0.2mol/LNaOH��Һ/mL | H2O /mL | Ʒ�� ��Һ | ���� |
�� | 5.0 | 0 | 0 | x | 4�� | �Ͽ���ɫ |
�� | 0 | 5.0 | 5.0 | 0 | 4�� | ����ɫ |
�� | 5.0 | 0 | 5.0 | 0 | 4�� | ������ɫ |
��x=_______�����ۣ�________________________________________________��