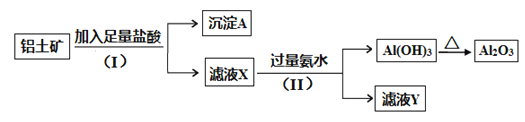
��Ŀ����
����Ŀ����ҵ����һ�ַ�����Ч�ؿ�������CO2������CO2������ȼ�ϼ״���Ϊ̽����Ӧԭ������������ʵ�飬�����Ϊ1L�ĺ����ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
CH3OH��g��+H2O��g������H=-49.0kJ/mol�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
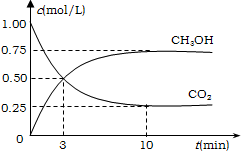
��1���ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��CO2��= ________mol/��Lmin����
��2��������ת����= ________��
��3������¶��¸÷�Ӧ��ƽ�ⳣ��K=________��
��4�����д�ʩ����ʹƽ����ϵ��n��CH3OH��/n��CO2���������________
A����H2O��g������ϵ�з����ȥ
B������He��g����ʹ��ϵѹǿ����
C�������¶�
D���ٳ���1mol CO2��3mol H2
��5������Ӧ�ﵽƽ��ʱ��CO2�����ʵ���Ũ��Ϊc1��Ȼ�����������ټ���һ����CO2������Ӧ��һ�δﵽƽ���CO2�����ʵ���Ũ��Ϊc2����c1________ c2����������������
���𰸡���1��0.075����2��75%����3��5.33����4��AD��5����
��������
�����������1����������ʽ���ⷨ����
CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g����
CH3OH��g��+H2O��g����
��ʼ��mol/L����1 3 0 0
�仯��mol/L����0.75 2.25 0.75 0.75
ƽ����mol/L����0.25 0.75 0.75 0.75
�ӷ�Ӧ��ʼ��ƽ����������ƽ����Ӧ����v��H2��=![]() =0.225 molL-1min-1��
=0.225 molL-1min-1��
���v��CO2��=![]() v��H2��=
v��H2��=![]() ��0.225 molL-1min-1=0.075 molL-1min-1���ʴ�Ϊ��0.075��
��0.225 molL-1min-1=0.075 molL-1min-1���ʴ�Ϊ��0.075��
��2��������ת����=![]() ��100%=75%���ʴ�Ϊ��75%��
��100%=75%���ʴ�Ϊ��75%��
��3��ƽ�ⳣ�������������Ũ����֮�����Է�Ӧ���Ũ����֮������
k=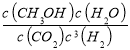 =
=![]() =5.33���ʴ�Ϊ��5.33��
=5.33���ʴ�Ϊ��5.33��
��4��Ҫʹn��CH3OH��/n��CO2������Ӧʹƽ��������Ӧ�����ƶ���A����H2O��g������ϵ�з��룬ƽ��������Ӧ�����ƶ���n��CH3OH��/n��CO2������A��ȷ��B������He��g����ʹ��ϵѹǿ�����Է�Ӧ������˵��Ũ��û�б仯��ƽ�ⲻ�ƶ���n��CH3OH��/n��CO2�����䣬��B����C��������Ӧ���ȣ������¶�ƽ�����淴Ӧ�����ƶ�����n��CH3OH��/n��CO2����С����C����D���ٳ���1mol CO2��3mol H2����Ч����ԭ����������С���һ�룬ѹǿ����ƽ��������Ӧ�����ƶ�����n��CH3OH��/n��CO2������D��ȷ���ʴ�Ϊ��AD��
��5�����������ټ���һ����CO2��c��CO2��������ƽ�������ƶ�����c1�� c2���ʴ�Ϊ������

 ��У����ϵ�д�
��У����ϵ�д�