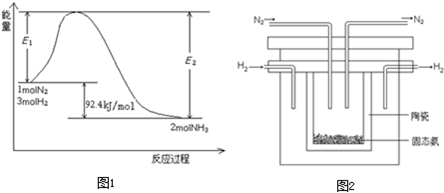
��Ŀ����
��2011?��ͷ����ģ�⣩��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��A
B
C
��1����A��ʹʪ��ĺ�ɫʯ����ֽ������CΪ����ɫ���壮��Aת��ΪB��Ӧ�Ļ�ѧ����ʽΪ
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ���C�ĵ���ʽΪ
 ��
��
��3����D�ǽ�����C��Һ������ʱӦ��������D���������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ˵����
��4����D��һ�ֳ������������壻A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӣ���Bת��ΪC�����ӷ���ʽΪ
��5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ���C��Һ�г�����������ڵ������ӵĻ�ѧʽΪ
��6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ������ʣ���B���ӵĽṹʽΪ
��7����A���������Ϊ75%����Һ������ҽ����������B������������ͭ���ȣ���ש��ɫ�������ɣ���A����B�Ļ�ѧ����ʽΪ
| +D |
| һ������ |
| +D |
| һ������ |
��1����A��ʹʪ��ĺ�ɫʯ����ֽ������CΪ����ɫ���壮��Aת��ΪB��Ӧ�Ļ�ѧ����ʽΪ
4NH3+5O2
4NO+6H2O
| ||
| �� |
4NH3+5O2
4NO+6H2O
��
| ||
| �� |
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ���C�ĵ���ʽΪ


��3����D�ǽ�����C��Һ������ʱӦ��������D���������ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ˵����
��������������ֹFe2+������ΪFe3+��2Fe3++Fe�T3Fe2+
��������������ֹFe2+������ΪFe3+��2Fe3++Fe�T3Fe2+
����4����D��һ�ֳ������������壻A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӣ���Bת��ΪC�����ӷ���ʽΪ
CO2+CO32-+H2O=2HCO3-
CO2+CO32-+H2O=2HCO3-
����5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ���C��Һ�г�����������ڵ������ӵĻ�ѧʽΪ
AlO2-
AlO2-
����6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ������ʣ���B���ӵĽṹʽΪ
O=C=O
O=C=O
����7����A���������Ϊ75%����Һ������ҽ����������B������������ͭ���ȣ���ש��ɫ�������ɣ���A����B�Ļ�ѧ����ʽΪ
2CH3CH2OH+O2
2CH3CHO+2H2O
| ���� |
| ���� |
2CH3CH2OH+O2
2CH3CHO+2H2O
��| ���� |
| ���� |
��������1����A��ʹʪ��ĺ�ɫʯ����ֽ�����ƶ�ΪNH3��CΪ����ɫ����ΪNO2���ж�DΪO2��BΪNO��
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ��ж�AΪNa
Na2O
Na2O2��
��3����D�ǽ�����C��Һ������ʱӦ��������D���ƶ�AΪCl2
FeCl3
FeCl2���������ǣ���������������ֹFe2+������ΪFe3+��2Fe3++Fe�T3Fe2+��
��4����D��һ�ֳ��������������ƶ�ΪCO2��A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӽ�����ӽṹ�ƶ�AΪNaOH
Na2CO3
NaHCO3��
��5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ����ж�ת����ϵΪAlCl3
Al��OH��3
NaAlO2��
��6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ��������ƶ�ת����ϵΪ��CuO
CO2
CO��
��7����A���������Ϊ75%����Һ������ҽ���������ж�Ϊ�Ҵ���B������������ͭ���ȣ���ש��ɫ�������ɣ�˵��Ϊ��ȩ��ת����ϵΪ��CH3CH2OH
CH3CHO
CH3COOH��
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ��ж�AΪNa
| O2 |
| O2 |
��3����D�ǽ�����C��Һ������ʱӦ��������D���ƶ�AΪCl2
| Fe |
| Fe |
��4����D��һ�ֳ��������������ƶ�ΪCO2��A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӽ�����ӽṹ�ƶ�AΪNaOH
| CO2 |
| CO2 |
��5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ����ж�ת����ϵΪAlCl3
| NaOH |
| NaOH |
��6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ��������ƶ�ת����ϵΪ��CuO
| C |
| C |
��7����A���������Ϊ75%����Һ������ҽ���������ж�Ϊ�Ҵ���B������������ͭ���ȣ���ש��ɫ�������ɣ�˵��Ϊ��ȩ��ת����ϵΪ��CH3CH2OH
| O2 |
| O2 |
����⣺��1����A��ʹʪ��ĺ�ɫʯ����ֽ�����ƶ�ΪNH3��CΪ����ɫ����ΪNO2���ж�DΪO2��BΪNO��Aת��ΪB��Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ��ж�AΪNa
Na2O
Na2O2��CΪNa2O2 ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3����D�ǽ�����C��Һ������ʱӦ��������D���ƶ�AΪCl2
FeCl3
FeCl2���ʴ�Ϊ����������������ֹFe2+������ΪFe3+��2Fe3++Fe�T3Fe2+��
��4����D��һ�ֳ��������������ƶ�ΪCO2��A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӽ�����ӽṹ�ƶ�AΪNaOH
Na2CO3
NaHCO3��Bת��ΪC�����ӷ���ʽΪ��CO2+CO32-+H2O=2HCO3-���ʴ�Ϊ��CO2+CO32-+H2O=2HCO3-��
��5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ����ж�ת����ϵΪAlCl3
Al��OH��3
NaAlO2����C��Һ�г�����������ڵ������ӵĻ�ѧʽΪAlO2-���ʴ�Ϊ��AlO2-��
��6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ��������ƶ�ת����ϵΪCuO
CO2
CO��B����ΪCO2�ĽṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
��7����A���������Ϊ75%����Һ������ҽ���������ж�Ϊ�Ҵ���B������������ͭ���ȣ���ש��ɫ�������ɣ�˵��Ϊ��ȩ��ת����ϵΪCH3CH2OH
CH3CHO
CH3COOH��A����B�Ļ�ѧ����ʽΪ2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��2����D�Ǿ��������Եĵ��ʣ�AԪ�����ڶ������������Ԫ�أ��ж�AΪNa
| O2 |
| O2 |
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3����D�ǽ�����C��Һ������ʱӦ��������D���ƶ�AΪCl2
| Fe |
| Fe |
��4����D��һ�ֳ��������������ƶ�ΪCO2��A��һ��ǿ���������ˮ��Һ�е���������������Ӿ�����10�����ӽ�����ӽṹ�ƶ�AΪNaOH
| CO2 |
| CO2 |
��5����DΪ�ȼҵ����Ҫ��Ʒ��B�������ԣ����ж�ת����ϵΪAlCl3
| NaOH |
| NaOH |
��6����A��B��C��Ϊ�����D��һ�ֺ�ɫ��̬�ǽ��������ƶ�ת����ϵΪCuO
| C |
| C |
��7����A���������Ϊ75%����Һ������ҽ���������ж�Ϊ�Ҵ���B������������ͭ���ȣ���ש��ɫ�������ɣ�˵��Ϊ��ȩ��ת����ϵΪCH3CH2OH
| O2 |
| O2 |
| ���� |
| ���� |
| ���� |
| ���� |
���������⿼�����������ʵ�Ӧ�ã���Ҫ���������ʣ���Ӧ����ת����ϵ�ķ����жϣ��������ջ����ǽ���ؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
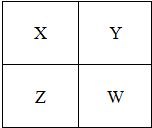 ��2011?��ͷ����ģ�⣩��֪X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������
��2011?��ͷ����ģ�⣩��֪X��Y��Z��W���ֶ�����Ԫ�������ڱ��е����λ����ͼ��ʾ������˵����ȷ���ǣ�������