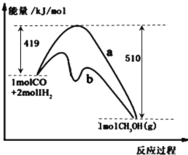
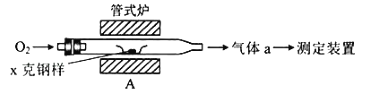
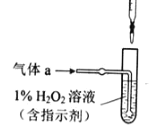
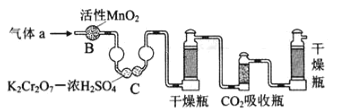
��Ŀ����
����Ŀ��Pd-Mg/SiO2������CO2���黯��Ӧ������ͼ��ʾ������˵��������ǣ� ��
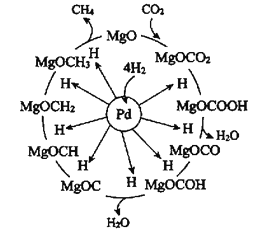
A.�������黯�����ܷ�Ӧ�ɱ�ʾΪCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)
CH4(g)+2H2O(g)
B.�������黯��������������õ�����ΪMgO
C.Pd-Mg/SiO2�����ӿ��˼��黯���ʣ������H2��ƽ��ת����
D.����һ������ѭ�����MgO�ٴ���CO2����γ�̼���Σ�����һ���µļ���ѭ������
���𰸡�BC
��������
A����ͼ��֪��CO2��H2��Pd-Mg/SiO2�����������ɼ����ˮ����ӦΪ��CO2+4H2![]() CH4+2H2O��A��ȷ��
CH4+2H2O��A��ȷ��
B���ɷ�Ӧ������֪CO2���黯��Ӧ�Ĺؼ����ڼ��⣬�������黯��������������õ�����ΪPd������
C���������Լӿ췴Ӧ���ʣ����������ƽ��ת���ʣ�C����
D��MgO��CO2����γ�̼���ξ���һ������ѭ�����ֵõ�MgO����ѧ����û�б仯�������μ��µļ���ѭ�����̣�D��ȷ��
��ѡBC��

����Ŀ����������:Ԫ�ظ�(Cr)����Һ����Ҫ��Cr3+(����ɫ) ��Cr(OH)4-(��ɫ) ��Cr2O72���Ⱥ�ɫ����CrO42����ɫ������ʽ���ڣ�Cr(OH)3Ϊ������ˮ�Ļ���ɫ���壬�ش��������⣺
��1����(24Cr)����________��ѡ���ţ���
a.����Ԫ�� b.����Ԫ�� c.����Ԫ�� d.����Ԫ��
��2��CrO42��Cr2O72����Һ�п��ת���������£���ʼŨ��Ϊ1.0 mol��L1��Na2CrO4��Һ��c��Cr2O72����c��H+���ı仯��ͼ��ʾ��

�������ӷ���ʽ��ʾNa2CrO4��Һ�е�ת����Ӧ____________��
����ͼ��֪����Һ��������CrO42��ƽ��ת����__________��������������С��������������������A�����ݣ��������ת����Ӧ��ƽ�ⳣ��Ϊ__________��
�������¶ȣ���Һ��CrO42��ƽ��ת���ʼ�С����÷�Ӧ����H_________0������>����<������=������
��3�������ͷ�ˮ���ؽ���Ԫ�ظ��Ķ��ԣ��ɽ�Cr2O72-ת��ΪCr(OH)3������ȥ����֪��
�������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
Fe2+ | 7.0 | 9.0 |
Fe3+ | 1.9 | 3.2 |
Cr3+ | 6.0 | 8.0 |
ij������ˮ��������Ҫ������ͼ��ʾ��
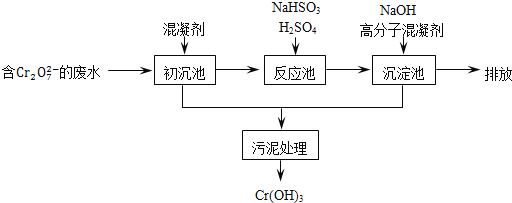
���������м���Ļ�������K2SO4�qAl2(SO4)3�q24H2O��������Ϊ__________�������ӷ���ʽ��ʾ����
����Ӧ������NaHSO3�����Է�Һ�е�Cr2O72-��ԭ��Cr3+���÷�Ӧ�����ӷ���ʽΪ________���������������������кͷ�����ԭ������������м���NaOH��Һ���˹����з�����Ҫ��Ӧ�����ӵ��Ⱥ�˳����____________��֤��Cr3+������ȫ�ķ�����__________��
��4����ҵ���õ�ⷨ�������� Cr2O72-��ˮ��ʵ����������ͼģ�����Cr2O72-�ķ�ˮ��������Ӧʽ��Fe-2e-�TFe2+��������Ӧʽ��2H++2e-�TH2����Fe2+��������Һ�е�Cr2O72-��Ӧ�����ӷ���ʽ��__________���õ��Ľ������������������ɳ�����ȫ���õ�ⷨ��������Һ��0.01mol Cr2O72-ʱ�����ٵõ�������������__________ g��

����Ŀ��ijС�����ʵ�飺�������ữ�Ĺ���������Һ�м���⻯�ء����ۺ���������ƵĻ����Һ��һ��ʱ�����Һ��������������֪��ϵ�д���������Ҫ��Ӧ��
��Ӧi��H2O2(aq) + 2I��(aq) + 2H+(aq) I2(aq) + 2H2O(l) ��H1 = -247.5 kJ/mol
��Ӧii��I2(aq) + 2S2O32-(aq) 2I��(aq) + S4O62-(aq) ��H2 = -1021.6 kJ/mol
��1��H2O2��S2O32-��Ӧ���Ȼ�ѧ����ʽΪ______��
��2������ʵ�鷽����֤ʵ������Ӧ���̡���ʵ�鷽�����������������Լ�Ũ�Ⱦ�Ϊ0.01 mol/L����
a�����ữ��H2O2��Һ�м���________��Һ����Һ������Ϊ��ɫ��
b��_________����Һ������ɫ��
��3��̽��c(H+)�Է�Ӧ���ʵ�Ӱ�죬ʵ�鷽�����±���ʾ���������Լ���H2O���⣬Ũ�Ⱦ�Ϊ0.01 mol/L��
ʵ����� | a | b | |
�� �� | H2O2/mL | 5 | ______ |
H2SO4/mL | 4 | 2 | |
Na2S2O3/mL | 8 | _____ | |
KI�������ۣ�/mL | 3 | _____ | |
H2O | 0 | ______ | |
��������ҺѸ�ٻ�� �۲����� | ��Һ��������ʱ�� Ϊt1�� | ��Һ��������ʱ�� Ϊt2�� | |
�� ��ʵ��b����������
�� �Ա�ʵ��a��ʵ��b��t1_____t2������>������<������
�� ��ϣ�2�������������Һ��Ϻ�һ��ʱ��ű�����ԭ��________��
�� ����ʵ��a�����ݣ�����t1ʱ����H2O2��S2O32-��Ӧ��ƽ����Ӧ���ʣ���H2O2Ũ�ȵı仯��ʾ��________mol/(L��s)��