��Ŀ����
ijѧ���������ʵ��װ��������ȡ��������ˮ�Ȼ�ͭ�����ݸ�С���Ҫ����д���пհף�
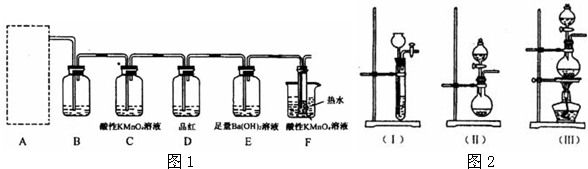
��1��װ��B��������
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��3��װ��E��������

��1��װ��B��������
��ȥCl2�л��е�HCl
��ȥCl2�л��е�HCl
��װ��C����������ȥCl2�л��е�ˮ����
��ȥCl2�л��е�ˮ����
����2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O
MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O
��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪCu+Cl2?CuCl2
Cu+Cl2?CuCl2
����3��װ��E��������
���ն����Cl2����ֹ��Ⱦ����
���ն����Cl2����ֹ��Ⱦ����
����������1������ʵ������ȡ�����ij��ӷ���˼������2������ʵ������ȡ������Ӧԭ���ͻ�ѧ���ʷ�������3�����������Ļ�ѧ���ʺ�β���Ĵ����������
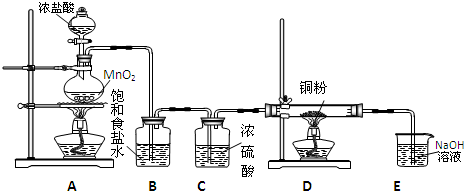
����⣺��1����ȡ����ʱ��Ҫ���ȣ���ʹŨ����ӷ����������Ҫ��װ��B��ȥHCl������ʹ�ñ���ʳ��ˮ������ȥHCl�ֽ���������ˮ�е��ܽ�ȣ���������������װ��Bʱ����ʹ�����л���ˮ������Ҫ��װ��C�е�Ũ��������ˮ������
��2��ʵ������ȡ������Ҫ��Ũ����Ͷ������̷�Ӧ����������������ԭ��Ӧ������ѧ����ʽΪ��MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O��װ��D���Ǵ����������������ͭ��Ӧ�����Ȼ�ͭ������ѧ����ʽΪ��Cu+Cl2?CuCl2��
��3����������ͭ��Ӧ����һ��������û�в��뷴Ӧ��ֱ���ŷŴ������л���ɻ�����Ⱦ�����Ҫ������������Һ�������Ȼ��ƺʹ������ƣ�������������ֹ��Ⱦ������
�ʴ�Ϊ��
��1����ȥCl2�л��е�HCl����ȥCl2�л��е�ˮ������
��2��MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O�� Cu+Cl2?CuCl2��
��3�����ն����Cl2����ֹ��Ⱦ������
��2��ʵ������ȡ������Ҫ��Ũ����Ͷ������̷�Ӧ����������������ԭ��Ӧ������ѧ����ʽΪ��MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O��װ��D���Ǵ����������������ͭ��Ӧ�����Ȼ�ͭ������ѧ����ʽΪ��Cu+Cl2?CuCl2��
��3����������ͭ��Ӧ����һ��������û�в��뷴Ӧ��ֱ���ŷŴ������л���ɻ�����Ⱦ�����Ҫ������������Һ�������Ȼ��ƺʹ������ƣ�������������ֹ��Ⱦ������
�ʴ�Ϊ��
��1����ȥCl2�л��е�HCl����ȥCl2�л��е�ˮ������
��2��MnO2+4HCl��Ũ��?MnCl2+Cl2��+2H2O�� Cu+Cl2?CuCl2��
��3�����ն����Cl2����ֹ��Ⱦ������
���������⿼����ǻ���ʵ���еĻ���֪ʶ��Ҫ��ѧϰ������ȡʱҪ����������������⣬���ܺ�ʵ�����������ں���ѧϰ��

��ϰ��ϵ�д�
 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
 ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�
ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�