题目内容
【题目】实验室从碘的四氯化碳溶液中分离并得到单质碘,主要步骤为:用浓NaOH溶液进行反萃取(3I2+6OH-=5I-+IO![]() +3H2O)、分液、酸化(5I-+IO
+3H2O)、分液、酸化(5I-+IO![]() +6H+=3I2↓+3H2O)、过滤及干燥等。下列有关实验原理和装置不能达到实验目的的是( )
+6H+=3I2↓+3H2O)、过滤及干燥等。下列有关实验原理和装置不能达到实验目的的是( )
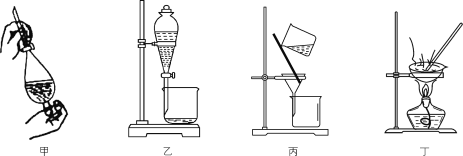
A.用装置甲反萃取时,倒转振荡过程中要适时旋开活塞放气
B.用装置乙分液时,先放出有机相,关闭活塞,从上口倒出水相
C.用装置丙从酸化后的体系中分离出单质碘
D.用装置丁干燥单质碘
【答案】D
【解析】
A. 用装置甲反萃取时,倒转振荡过程中要适时旋开活塞放气,避免内部压强过大,A正确;
B. 用装置乙分液时,先放出有机相,关闭活塞,从上口倒出水相,可以避免上层液体被污染,B正确;
C. 大量析出的固体碘单质用过滤分离出来,C正确;
D. 碘易升华,不能达到实验目的,D错误;
答案选D。

 阳光试卷单元测试卷系列答案
阳光试卷单元测试卷系列答案【题目】饮水安全在灾后重建中具有极其重要的地位,某研究小组提取三处被污染的水源进行了如下分析,给出了如下实验信息:其中一处被污染的水源含有A、B两种物质,一处含有C、D两种物质、一处含有E物质,A、B、C、D、E五种常见化合物,都是由下表中的离子形成的:
阳离子 | K+ Na+ Cu2+ Al3+ |
阴离子 | SO |
为了鉴别上述化合物,分别完成以下实验,其结果是:
①将它们溶于水后,D为蓝色溶液,其他均为无色溶液;
②将E溶液滴入到C溶液中出现白色沉淀,继续滴加,沉淀溶解;
③进行焰色反应,只有B、C为紫色(透过蓝色钴玻璃);
④在各溶液中加入硝酸钡溶液,再加过量稀硝酸,A中放出无色气体,C、D中产生白色沉淀;
⑤将B、D两溶液混合,未见沉淀或气体生成。
根据上述实验填空:
(1)写出B、D的化学式:B__,D__。
(2)将含1molA的溶液与含1molE的溶液充分反应,仅得到一种化合物,该化合物为__。(填化学式)
(3)写出实验②发生反应的离子方程式__。
(4)C常用作净水剂,用离子方程式表示其净水原理__。
【题目】室温下进行下列实验,根据实验操作和现象所得到的结论正确的是( )
选项 | 实验操作和现象 | 结论 |
A | 向NaHSO3溶液中滴加足量Ba(OH)2溶液,出现白色沉淀,再加入足量盐酸,沉淀全部溶解 | NaHSO3未被氧化 |
B | 向3mLFe(NO3)3溶液中滴加几滴HI溶液,振荡,再滴加1mL淀粉溶液,溶液显蓝色 | I-的还原性比Fe2+的强 |
C | 向MgSO4、CuSO4的混合稀溶液中滴入1滴稀NaOH溶液,有蓝色沉淀生成 | Ksp[Cu(OH)2]<Ksp[Mg(OH)2] |
D | 用精密pH试纸测得:浓度均为0.1mol·L-1的NH4HCO3溶液、HCOONa溶液的pH分别为7.8、10.0 | H2CO3电离出H+的能力比HCOOH的强 |
A.AB.BC.CD.D


