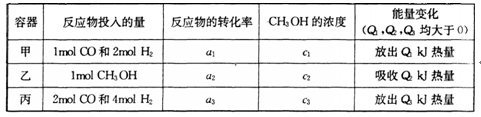
��Ŀ����
��֪��N2(g)��3H2(g)  2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ���� :
2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ���� :
 2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ���� :
2NH3(g)����H����Q kJ��mol��1��Q��0�������м���������ͬ���ܱ���������������г���1mol N2(g)��3mol H2(g)����һ�������´ﵽƽ��ʱ�ų�������ΪQ1 kJ������ͬ���������������г���2mol NH3(g)���ﵽƽ��ʱ���յ�����ΪQ2 kJ����֪Q2��3Q1��������������ȷ���� :| A��ƽ��ʱ��������NH3(g)������������������е�С |
| B��ƽ��ʱ�������������ѹǿΪ��ʼʱѹǿ�� |
| C���ﵽƽ��ʱ����������H2��ת����Ϊ25�� |
| D��Q1��Q |
C
�¶���ͬ�����������ͬ���һ�Ϊ��Чƽ��
����������N2�μӷ�Ӧxmol��
N2(g)��3H2(g) 2NH3(g)����H����Q kJ��mol��1
2NH3(g)����H����Q kJ��mol��1
��ʼʱ����(moi) �� 1 3 0
ת������(mol) �� X 3x 2x
ƽ��ʱ����(mol)�� 1-x 3-3x 2x
�����Ϊ��Чƽ�⣬��ƽ��ʱNH3Ϊ2xmol
N2(g)��3H2(g) 2NH3(g)����H����Q kJ��mol��1
2NH3(g)����H����Q kJ��mol��1
��ʼʱ����(mol): 0 0 2
ת������(mol): 0.5(2-2x) 1.5(2-2x) 2-2x
ƽ��ʱ����(mol):0.5(2-2x) 1.5(2-2x�� 2x
�ų�������Ϊ��Q2=(2-2X)��Q��2
��(2-2X)��Q��2=3Q��2��2x
X=o.25
�����ϼ����֪������Ϊ��Чƽ��NH3(g)�����������ͬ��A����ƽ��ʱ�������ʵ���Ϊ3.5mol����ʼΪ4mol,��Ϊ 7/8��B����ƽ��ʱ����H2�μӷ�ӦΪ0.75mol,��ʼΪ3mol����C�ԡ�����N2�μӷ�ӦΪ0.25mol����Q1=0.5Q��D����
7/8��B����ƽ��ʱ����H2�μӷ�ӦΪ0.75mol,��ʼΪ3mol����C�ԡ�����N2�μӷ�ӦΪ0.25mol����Q1=0.5Q��D����
����������N2�μӷ�Ӧxmol��
N2(g)��3H2(g)
 2NH3(g)����H����Q kJ��mol��1
2NH3(g)����H����Q kJ��mol��1��ʼʱ����(moi) �� 1 3 0
ת������(mol) �� X 3x 2x
ƽ��ʱ����(mol)�� 1-x 3-3x 2x
�����Ϊ��Чƽ�⣬��ƽ��ʱNH3Ϊ2xmol
N2(g)��3H2(g)
 2NH3(g)����H����Q kJ��mol��1
2NH3(g)����H����Q kJ��mol��1��ʼʱ����(mol): 0 0 2
ת������(mol): 0.5(2-2x) 1.5(2-2x) 2-2x
ƽ��ʱ����(mol):0.5(2-2x) 1.5(2-2x�� 2x
�ų�������Ϊ��Q2=(2-2X)��Q��2
��(2-2X)��Q��2=3Q��2��2x
X=o.25
�����ϼ����֪������Ϊ��Чƽ��NH3(g)�����������ͬ��A����ƽ��ʱ�������ʵ���Ϊ3.5mol����ʼΪ4mol,��Ϊ
 7/8��B����ƽ��ʱ����H2�μӷ�ӦΪ0.75mol,��ʼΪ3mol����C�ԡ�����N2�μӷ�ӦΪ0.25mol����Q1=0.5Q��D����
7/8��B����ƽ��ʱ����H2�μӷ�ӦΪ0.75mol,��ʼΪ3mol����C�ԡ�����N2�μӷ�ӦΪ0.25mol����Q1=0.5Q��D����
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
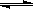 2NO(g)��ƽ�ⳣ��K���±���
2NO(g)��ƽ�ⳣ��K���±���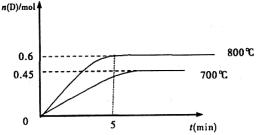
 2SO3(g)����H=��akJ��mo1��2����ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������( )
2SO3(g)����H=��akJ��mo1��2����ͬ������Ҫ��õ�2akJ��������������ʵ����ʵ���������( ) �������ͼʾ�ش��������⣺
�������ͼʾ�ش��������⣺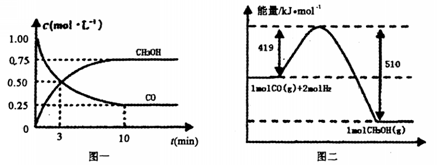
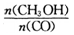 �������________________
�������________________
 N2O4(g) ��H��0�������¶ȸ÷�Ӧƽ�ⳣ������
N2O4(g) ��H��0�������¶ȸ÷�Ӧƽ�ⳣ������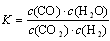 �ķ�Ӧ����ѧ����ʽΪCO2��H2
�ķ�Ӧ����ѧ����ʽΪCO2��H2 CO��H2O
CO��H2O 2NH3(g) ��H��0,��Ӧ��NH3�����ʵ���Ũ�ȵı仯���������ͼ��
2NH3(g) ��H��0,��Ӧ��NH3�����ʵ���Ũ�ȵı仯���������ͼ��
 2SO3��g��������ӦΪ���ȷ�Ӧ�������д�ʩ�У��ȿɼӿ췴Ӧ���ʣ��ֿ�ʹƽ�������ƶ�����
2SO3��g��������ӦΪ���ȷ�Ӧ�������д�ʩ�У��ȿɼӿ췴Ӧ���ʣ��ֿ�ʹƽ�������ƶ�����