��Ŀ����
��������Խ����Ⱥ͵�֮�䣬������һ���ʽ����������⡣
(1)����Ϊ���������������ַ��ӻ����ӵ��Լ����뵽����Br������Һ�У����Խ�Br������ΪBr2__________��
| A��I2 | B��I | C��Cl2 | D��Cl�� |
(3)����ֽ�õ��ۺ͵⻯�صĻ����Һ���ݣ����ɺ����ʵ���ҳ��õĵ��۵�
������ֽ��������ֽ��ʪ���������������ı仯��_____________��ԭ����_________________________________________��
(1)C��(2)I������ԭ��I��>Br��
(3)��ֽ����ɫ��Cl2��I����������I2��I2��������ñ���ɫ
����

��ϰ��ϵ�д�
�����Ŀ
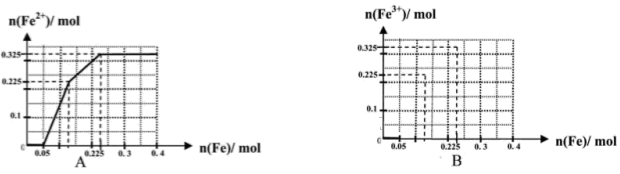
��һ����8�֣��ں������ʵ�����Ϊ0.1 mol FeCl3��H2SO4��Cu(NO3)2����Һ500 mL�У��������ۣ���Һ��n(Fe2��)�����n(Fe)�Ĺ�ϵ��ͼA(��ע��ʶͼ������ͼ��ÿС������Ϊ0.05mol)������ʾ����������ˮ�������Ӱ�죻��������������ԭʱ��������һ���������壩
��1��д��ͼ��n(Fe)��0.125mol-0.225 mol�ζ�Ӧ��Ӧ�����ӷ���ʽ____��
��2��д��ͼ��n(Fe)��0-0.05 mol�ζ�Ӧ��Ӧ�����ӷ���ʽ____��
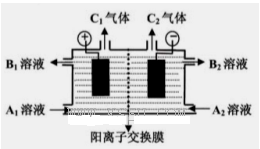
��3������ͼB�л�����Һ��n(Fe3��)�����n(Fe)�ı仯��ϵͼ
��4������Ӧ���е����ȡ����Һ2mL�����Թ��У�Ϊ��֤������Һ����NO3�����ڣ��������Թ����ڵμ�_______��
| A������KMnO4��Һ | B�����������KSCN��Һ | C����ˮ | D������������Һ |
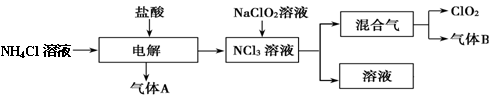
��������5�֣���ͼ�ǵ��۵�����ͼ����ͷ�ķ���ָ�����������Һ�����������ӽ���Ĥֻ����������ͨ������Ҫ�ô�װ����ͨ��������Na2SO4��NaOH�Ļ����Һ������װ����ͨ��A1��Һ��A2��Һ(A1��Һ��Na2SO4��NaOH�Ļ����Һ��A2��Һ��NaOH��ϡ��Һ)�����һ��ʱ���װ����������B2��Һ����NaOH��Ũ��Һ����B1��Һ��C1�����C2���塣
��1�� C2������______���ѧʽ����
��2����д��������Ӧ�ĵ缫����ʽ .
��3�������������������NaOH��Ũ��Һ��ԭ�� .
ijѧ����һ֧�Թ��ﰴһ������ֱ�������м������ʣ�
| A��KI��Һ�� | B��������Һ�� | C��NaOH��Һ�� | D����ˮ��������Һ��ɫ���������仯�� |
������Һ��ɫ�ı仯�ش��������⣺
��1����������ҩƷ��˳����_________________________________________________��
��2��д���١��ڵ����ӷ�Ӧ����ʽ����Ϊ������ԭ��Ӧ�����������ת�Ƶķ����������_______________________________________________________________________��
��3��д���ۡ��ܵĻ�ѧ��Ӧ����ʽ��___________________________________________��
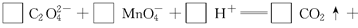
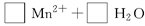
 Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�: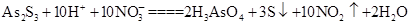 ��������2 mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ����______����������������������ݳ���
��������2 mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ����______����������������������ݳ���