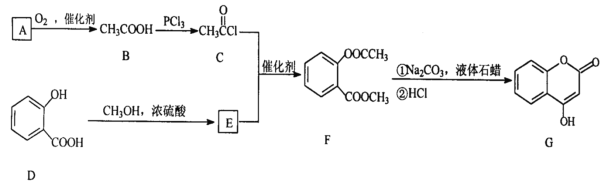
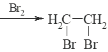ЬтФПФкШн
ЁОЬтФПЁПЯТЭМЪЧвЛИіЕчЛЏбЇЙ§ГЬЕФЪОвтЭМЃЌЧыАДвЊЧѓЬюПеЃК
(1)ЭЈШыCH3CH2OHЕФЕчМЋУћГЦЪЧ______________ЃЌBЕчМЋЕФУћГЦЪЧ_____________ЁЃ
(2)ЭЈШыCH3CH2OHвЛМЋЕФЕчМЋЗДгІЪНЮЊ_____________________________________ЁЃ
(3)ввГижазмЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________________________________________ЁЃ
(4)ЕБввГижаA(Fe)МЋЕФжЪСПдіМг12.8gЪБЃЌМзГижаЯћКФO2 _______ mL(БъПіЯТ)
(5)Л№М§ЗЂЩфЪБПЩгУыТ(N2H4)ЮЊШМСЯЃЌвдЖўбѕЛЏЕЊзібѕЛЏМСЃЌЫќУЧЯрЛЅЗДгІЩњГЩЕЊЦјКЭЫЎеєЦјЁЃ
ЂйаДГідкМюадЬѕМўЯТИКМЋЗДгІЪНЮЊЃК___________________________________________ЁЃ
ЂквбжЊЃКN2(g)ЃЋ2O2(g)=2NO2(g)ЃЌІЄHЃНЃЋ67.7 kJЁЄmolЃ1ЃЛN2H4(g)ЃЋO2(g)=N2(g)ЃЋ2H2O(g)ЃЌІЄHЃНЃ534 kJЁЄmolЃ1ЃЌдђN2H4КЭNO2ЗДгІЕФШШЛЏбЇЗНГЬЪН__________________________________________ЁЃ
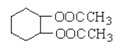
ЁОД№АИЁПИКМЋ бєМЋ C2H5OH-12e-+16OH-=2CO![]() +11H2O 2CuSO4+2H2O
+11H2O 2CuSO4+2H2O![]() 2Cu+O2Ёќ+2H2SO4 2240 N2H4-4e-+ 4OHЁЅ=N2+4H2O 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g)ЁїH=-1135.7kJmol-1
2Cu+O2Ёќ+2H2SO4 2240 N2H4-4e-+ 4OHЁЅ=N2+4H2O 2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g)ЁїH=-1135.7kJmol-1
ЁОНтЮіЁП
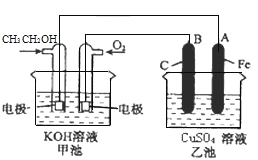
МзГиЮЊввДМШМСЯЕчГиЃЌЗХЕчЙ§ГЬжаввДМБЛбѕЛЏзїИКМЋЃЌгЩгкЕчНтжЪШмвКЯдМюадЃЌЫљвдЩњГЩCO![]() КЭЫЎЃЛбѕЦјБЛЛЙдзїе§МЋЃЌМюадЕчНтжЪШмвКжаЩњГЩOHЁЅЃЛввГиЮЊЕчНтГизАжУЃЌBЕчМЋгыдЕчГие§МЋЯрСЌЮЊбєМЋЃЌЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃЌAЕчМЋгыдЕчГиИКМЋЯрСЌЮЊвѕМЋЃЌЕУЕчзгЗЂЩњЛЙдЗДгІЁЃ
КЭЫЎЃЛбѕЦјБЛЛЙдзїе§МЋЃЌМюадЕчНтжЪШмвКжаЩњГЩOHЁЅЃЛввГиЮЊЕчНтГизАжУЃЌBЕчМЋгыдЕчГие§МЋЯрСЌЮЊбєМЋЃЌЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃЌAЕчМЋгыдЕчГиИКМЋЯрСЌЮЊвѕМЋЃЌЕУЕчзгЗЂЩњЛЙдЗДгІЁЃ
(1)ЭЈШыввДМЕФЕчМЋЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃЌЮЊдЕчГиЕФИКМЋЃЛBЕчМЋгыдЕчГие§МЋЯрСЌЮЊбєМЋЃЛ
(2)ввДМБЛбѕЛЏзїИКМЋЃЌгЩгкЕчНтжЪШмвКЯдМюадЃЌЫљвдЩњГЩCO![]() КЭЫЎЃЌЕчМЋЗНГЬЪНЮЊC2H5OH-12e-+16OHЁЅ=2CO
КЭЫЎЃЌЕчМЋЗНГЬЪНЮЊC2H5OH-12e-+16OHЁЅ=2CO![]() +11H2OЃЛ
+11H2OЃЛ
(3)ввГижабєМЋЩЯЫЎЕчРыГіЕФЧтбѕИљЗХЕчЩњГЩбѕЦјЃЌЭЌЪБВњЩњЧтРызгЃЌвѕМЋЩЯЭРызгЗХЕчЩњГЩЭЕЅжЪЃЌЫљвдЕчГизмЗДгІЕФЛЏбЇЗНГЬЪНЮЊ2CuSO4+2H2O![]() 2Cu+O2Ёќ+2H2SO4ЃЛ
2Cu+O2Ёќ+2H2SO4ЃЛ
(4)ввГижаAЕчМЋЮЊвѕМЋЃЌЕчМЋЗДгІЮЊCu2++2e-=CuЃЌЮіГіЭЕФЮяжЪЕФСПЮЊ![]() =0.2molЃЌЫљвдзЊвЦЕФЕчзгЮЊ0.4molЃЌМзГижаЭЈШыбѕЦјЕФвЛМЋЕФЕчМЋЗДгІЮЊO2+2H2O+4e-=4OHЁЅЃЌЫљвдзЊвЦ0.4molЕчзгЪБЯћКФ0.1molбѕЦјЃЌБъПіЯТЬхЛ§ЮЊ0.1molЁС22.4L/mol=2.24L=2240mLЃЛ
=0.2molЃЌЫљвдзЊвЦЕФЕчзгЮЊ0.4molЃЌМзГижаЭЈШыбѕЦјЕФвЛМЋЕФЕчМЋЗДгІЮЊO2+2H2O+4e-=4OHЁЅЃЌЫљвдзЊвЦ0.4molЕчзгЪБЯћКФ0.1molбѕЦјЃЌБъПіЯТЬхЛ§ЮЊ0.1molЁС22.4L/mol=2.24L=2240mLЃЛ
(5)ЂйдЕчГижаИКМЋЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃЌИУЕчГижаN2H4БЛNO2бѕЛЏЃЌЫљвдN2H4ЮЊИКМЋдСЯЃЌЕчНтжЪШмвКЯдМюадЃЌЫљвдЕчМЋЗНГЬЪНЮЊN2H4-4e-+ 4OHЁЅ=N2+4H2OЃЛ
ЂквбжЊiЃКN2(g)+2O2(g)=2NO2(g)ЃЌЁїH=+67.7kJmol-1ЃЛ
iiЃКN2H4(g)+O2(g)=N2(g)+2H2O(g)ЃЌЁїH=-534kJmol-1
ИљОнИЧЫЙЖЈТЩЃЌ2ЁСЂк-ЂйПЩЕУ2N2H4(g)+2NO2(g)=3N2(g)+4H2O(g) ЁїH=2(-534kJmol-1)-(+67.7kJmol-1)=-1135.7kJmol-1ЁЃ

 ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ
ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ ЭЌВНЭиеЙдФЖСЯЕСаД№АИ
ЭЌВНЭиеЙдФЖСЯЕСаД№АИЁОЬтФПЁПXЁЂYСНИљН№ЪєАєВхШыZШмвКжаЙЙГЩШчЭМЕФзАжУЃЌЪЕбщжаЕчСїБэжИеыЗЂЩњЦЋзЊЃЌЭЌЪБXАєБфДжЃЌYАєБфЯИЃЌдђXЁЂYЁЂZПЩФмЪЧ( )
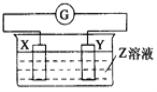
бЁЯю | X | Y | Z |
A | Zn | Cu | ЯЁСђЫс |
B | Cu | Zn | ЯЁСђЫс |
C | Cu | Ag | СђЫсЭШмвК |
D | Ag | Zn | ЯѕЫсвјШмвК |
A.AB. BC. CD. D