��Ŀ����
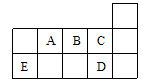
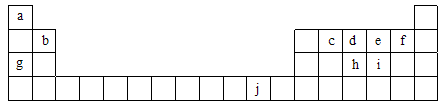
(16��)�±�Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
�û�ѧ����ش��������⣺
(1)д��Ԫ��f�Ļ�̬ԭ�Ӻ�������Ų�ʽ___________________________��
(2)��c6a6�����У�Ԫ��cΪ �ӻ����÷����� ����(����ԡ��Ǽ��ԡ�)��
(3)ci2���ӵĵ���ʽΪ_________________________��ci2��ce2�Ƚϣ��е�ϸߵ���_____________(д����ʽ)��
(4)��һ�����ܣ�h______i���縺�ԣ�g______b(�����������������)��
(5)���й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ�� ��
(6)����ˮ���뵽j����������Һ�У��Ȳ�����ɫ������Ȼ��������ܽⲢ�õ�����ɫ��Һ��������ɫ��������____________________��д����ɫ�����ܽ��ڰ�ˮ�е����ӷ���ʽ______________________________________________________________________��
(7)j�Ľ�������ľ�����ͼ��ʾ����һ��������jԭ�ӵĸ�����_______����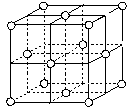

�û�ѧ����ش��������⣺
(1)д��Ԫ��f�Ļ�̬ԭ�Ӻ�������Ų�ʽ___________________________��
(2)��c6a6�����У�Ԫ��cΪ �ӻ����÷����� ����(����ԡ��Ǽ��ԡ�)��
(3)ci2���ӵĵ���ʽΪ_________________________��ci2��ce2�Ƚϣ��е�ϸߵ���_____________(д����ʽ)��
(4)��һ�����ܣ�h______i���縺�ԣ�g______b(�����������������)��
(5)���й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ�� ��
| A��jλ��Ԫ�����ڱ��е������ڡ���B�壬����ds��Ԫ�� |
| B��d�Ļ�̬ԭ���У�2p�ܼ�Ϊ�����������p��Ԫ�� |
| C�����������Ų�ʽΪ4s1��һ�����ڢ�A�� |
| D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B�� |
(7)j�Ľ�������ľ�����ͼ��ʾ����һ��������jԭ�ӵĸ�����_______����

(1) 1s22s22p5��2�֣� (2) sp2 �Ǽ��ԣ���1�֣�
(3)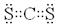 CS2����1�֣� (4) �� ������1�֣� (5) A B��2�֣�
CS2����1�֣� (4) �� ������1�֣� (5) A B��2�֣�
(6) [Cu(NH3)4]2����1�֣� Cu(OH)2��4NH3��H2O��[Cu(NH3)4]2����2OH����4H2O��3�֣�
(7) 4��2�֣�
(3)
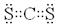 CS2����1�֣� (4) �� ������1�֣� (5) A B��2�֣�
CS2����1�֣� (4) �� ������1�֣� (5) A B��2�֣�(6) [Cu(NH3)4]2����1�֣� Cu(OH)2��4NH3��H2O��[Cu(NH3)4]2����2OH����4H2O��3�֣�
(7) 4��2�֣�
��Ԫ�������ڱ��е�λ�ÿ�֪a��j�ֱ���H��Be��C��N��O��F��Na��P��S��Cu��
��1�����ݹ���ԭ����֪F�Ļ�̬ԭ�Ӻ�������Ų�ʽ1s22s22p5��
��2���ں��ﱽ��ƽ���������νṹ������̼Ԫ����sp2�ӻ������ڷǼ��Է��ӡ�
��3��CS2���ɼ��Լ����ɵķǼ��Է��ӣ�����ʽΪ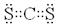 ��CO2��CS2���Ƿ��Ӿ��壬�е�ߵͺͷ��Ӽ���������С�й�ϵ��������Է���������CS2�Ĵ����Ե�CS2�е�ߡ�
��CO2��CS2���Ƿ��Ӿ��壬�е�ߵͺͷ��Ӽ���������С�й�ϵ��������Է���������CS2�Ĵ����Ե�CS2�е�ߡ�
��4��������ԭ�ӵ�3p������ڰ�������ȶ��Ժã���˵�һ�����ܴ���S�ġ�������Խ�����縺��ԽС�������Ƶĵ縺��С��Be�ġ�
��5��J��ͭ��A��ȷ����Ԫ�ص�2p���ڰ����������p����B��ȷ��C����ȷ������ͭ�����������Ų�ʽΪ4s1����ͭ���ڵ�IB�����������Ų�ʽΪns2np1��Ԫ��һ�����ڵڢ�A��D����ȷ�����Դ�ѡAB��
��6��������ͭ���ӿ��γ���λ�������ӷ���ʽΪCu(OH)2��4NH3��H2O��[Cu(NH3)4]2����2OH����4H2O��
(7)���ݾ����ṹͼ��֪�����е�ͭԭ����8��1/8��6��1/2��4��
��1�����ݹ���ԭ����֪F�Ļ�̬ԭ�Ӻ�������Ų�ʽ1s22s22p5��
��2���ں��ﱽ��ƽ���������νṹ������̼Ԫ����sp2�ӻ������ڷǼ��Է��ӡ�
��3��CS2���ɼ��Լ����ɵķǼ��Է��ӣ�����ʽΪ
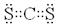 ��CO2��CS2���Ƿ��Ӿ��壬�е�ߵͺͷ��Ӽ���������С�й�ϵ��������Է���������CS2�Ĵ����Ե�CS2�е�ߡ�
��CO2��CS2���Ƿ��Ӿ��壬�е�ߵͺͷ��Ӽ���������С�й�ϵ��������Է���������CS2�Ĵ����Ե�CS2�е�ߡ���4��������ԭ�ӵ�3p������ڰ�������ȶ��Ժã���˵�һ�����ܴ���S�ġ�������Խ�����縺��ԽС�������Ƶĵ縺��С��Be�ġ�
��5��J��ͭ��A��ȷ����Ԫ�ص�2p���ڰ����������p����B��ȷ��C����ȷ������ͭ�����������Ų�ʽΪ4s1����ͭ���ڵ�IB�����������Ų�ʽΪns2np1��Ԫ��һ�����ڵڢ�A��D����ȷ�����Դ�ѡAB��
��6��������ͭ���ӿ��γ���λ�������ӷ���ʽΪCu(OH)2��4NH3��H2O��[Cu(NH3)4]2����2OH����4H2O��
(7)���ݾ����ṹͼ��֪�����е�ͭԭ����8��1/8��6��1/2��4��

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
�����Ŀ