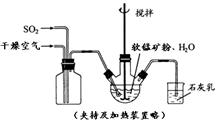
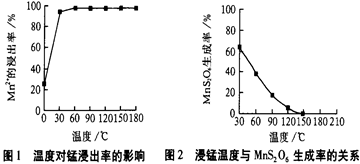
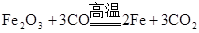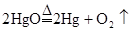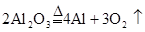��Ŀ����
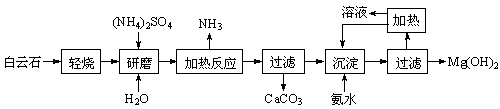
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
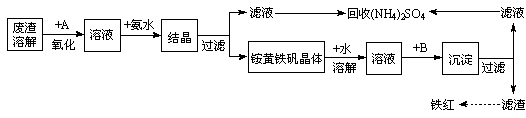
�ش��������⣺
��1���ڷ����ܽ����ʱ��Ϊ�˼��ٷ����ܽ�Ĵ�ʩ�ǣ�___________________��__________________����д���㣩
��2������A��һ��������
�ٹ�ҵ�����ѡ�� ������ţ�
�ڼ�����ҵ������ѡ��A�������� ��________________�������2������
��д��A���뷴Ӧ�����ӷ���ʽΪ__________________________________________
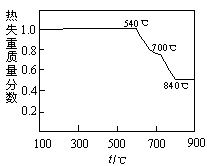
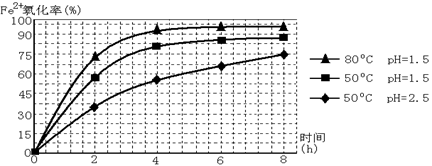
��3������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ�������__________________��
��4����炙�������Һ(��Fe3+)�м�����ҺB��pHΪ5ʱ������������д���������������ӷ���ʽ��_______________________________________________________��
��5��д��炙���������������������ˮ�У������м������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ��_____________________________________________________
��6���������õ�(NH4)2SO4������ܺ��е������ǣ�___________________________

�ش��������⣺
��1���ڷ����ܽ����ʱ��Ϊ�˼��ٷ����ܽ�Ĵ�ʩ�ǣ�___________________��__________________����д���㣩
��2������A��һ��������
�ٹ�ҵ�����ѡ�� ������ţ�
| A������ | B��Cl2 | C��MnO2 | D��H2O2�� |
��д��A���뷴Ӧ�����ӷ���ʽΪ__________________________________________
��3������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ�������__________________��

��4����炙�������Һ(��Fe3+)�м�����ҺB��pHΪ5ʱ������������д���������������ӷ���ʽ��_______________________________________________________��
��5��д��炙���������������������ˮ�У������м������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ��_____________________________________________________
��6���������õ�(NH4)2SO4������ܺ��е������ǣ�___________________________
��1�����Ͻ��裻���������飻�����¶ȣ��ʵ�����A��Ũ�ȵ� ��2�֣�
��2����A����������2�֣�
��ԭ����Դ���׳ɱ��ͣ���������Ⱦ�����������ʡ���2�֣����������㼴�ɣ�
��4Fe2++O2+4H+=4Fe3++2H2O ��2�֣�
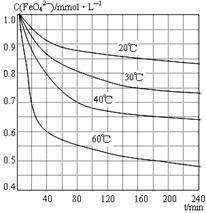
��3����Һ�¶ȿ�����80�棬pH������1.5��1�֣�������ʱ��Ϊ4Сʱ���ң�1�֣�
��4��Fe3++3NH3��H2O=Fe(OH)3��+3NH4+��2�֣�
��5��NH4++Fe3+2SO42-+2Ba2++2OH-=2BaSO4��+NH3.H2O+Fe(OH)3����2�֣�
��6��MgSO4��CaSO4��2�֣�
��2����A����������2�֣�
��ԭ����Դ���׳ɱ��ͣ���������Ⱦ�����������ʡ���2�֣����������㼴�ɣ�
��4Fe2++O2+4H+=4Fe3++2H2O ��2�֣�
��3����Һ�¶ȿ�����80�棬pH������1.5��1�֣�������ʱ��Ϊ4Сʱ���ң�1�֣�
��4��Fe3++3NH3��H2O=Fe(OH)3��+3NH4+��2�֣�
��5��NH4++Fe3+2SO42-+2Ba2++2OH-=2BaSO4��+NH3.H2O+Fe(OH)3����2�֣�
��6��MgSO4��CaSO4��2�֣�
�����������1���ӿ췴Ӧ���ʵ�������Ũ�ȡ��¶ȡ�����Ӵ�����ȣ���˼ӿ��ܽ����ʿ��Բ��Ͻ��衢���������顢�����¶ȡ��ʵ�����A��Ũ�ȵ� ��2�֣�
��2����Fe2+����ΪFe3+��ͨ����������ˣ����������ã����Խ�Լ�ɱ�����������Ⱦ�����������ӷ�Ӧ����ʽ�� 4Fe2++O2+4H+=4Fe3++2H2O ��2�֣�
��3������ͼ�е���������¶ȡ�ʱ�䡢pHֵ����˶Աȷ�����Һ��80�棬pH������1.5������ʱ��Ϊ4Сʱ����ʱ����Ч����ѡ�
��4���������̿�֪��������õ������������Ʊ����죬���Կ�֪������Fe(OH)3 ����ҺΪ(NH4)2SO4 �������pH���Լ�Ӧ��ѡ�ð�ˮ�������Ͳ����������������ӣ������ķ�ӦΪ��Fe3++3 NH3��H2O=Fe(OH)3��+3NH4+
��5��炙�����������������ϣ�NH4+��OH- ��Ӧ����NH3.H2O�� Fe3+��OH-����Fe(OH)3 ������SO42-��Ba2+����BaSO4�������������ӷ���ʽΪNH4++Fe3+2SO42-+2Ba2++2OH-=2BaSO4��+NH3.H2O+Fe(OH)3��
��6����Ϊ�������к���CaO��MgO�������ܽ��Ժ��ڽᾧ�õ�炙�����ʱ������õ�����Һû�г��ӣ���˻����MgSO4��CaSO4 �����ʡ� ��2�֣�

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ