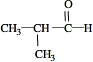
��Ŀ����
4������ʱ��8��12����23ʱ30�����ң������������������������ұ�ը���������α�ը�����Լ30�룬�ڶ��α�ը�������ߣ��൱��21��TNT����46öս��ʽѲ�����������µ����������������˾������ʾ���ù�˾�ִ�ҵ�����Ʒ����У�ѹ�������Һ�����壨�����ѹ����Ȼ���ȣ�����ȼҺ�壨����ͪ�����������ȣ�����ȼ���塢��ȼ��Ʒ����ʪ��ȼ��Ʒ�������ơ�����þ����ǡ�������ά�ء���ʯ����ƺϽ�ȣ������������л������������ء������Ƶȣ�������Ʒ���軯�ơ��ױ������������ȣ�����ʴƷ�����ࣨ���ᡢ���ᡢ�����ᡢ�ռ��ȣ��ȶ�����࣮��֪���軯�⣨HCN����״̬��ΪҺ�壮�軯�����ڿ����о�����ɢ���ڿ����п�ȼ�գ��軯���ڿ����еĺ����ﵽ5.6%��12.8%ʱ�����б�ը�ԣ����������ھ綾�࣮�����ᣨHCN����̼���������µĵ��볣���ֱ�Ϊ��HCN��Ka=6.2��10-10mol•L-1��
H2CO3��Ka1=4.4��10-7mol•L-1��Ka2=4.7��10-11mol•L-1
���û�ѧ����ʽ�ش����и��⣺
��1��������������ȼ�������£�44g����������ȫȼ�շų� 1122.1kJ��������д����������ȼ���� ���Ȼ�ѧ��Ӧ����ʽ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol��
��2����ȼ�������ƣ�����ȼ���Ż�ʱ���ֽܷ����һ�������������ƣ����û�ѧ����ʽ��ʾԭ��2NaNO3=2NaNO2+O2����
��3����ȼ��������ƣ���ֹ��ˮǹ��ˮ������û�ѧ����ʽ��ʾԭ��2Na+2H2O=2NaOH+H2����
��4�����軯�Ʒ�������ʱ����ֹ����ĭ������𣬷������ױ����������ɣ����û�ѧ����ʽ��ʾԭ��NaCN+CO2+H2O=HCN+NaHCO3��
��5�����������������ڡ����衱���軯�ƣ���Ӧ�����������ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3��
���� ��1��ȼ���ȵ��Ȼ�ѧ����ʽ�п�ȼ��Ϊ1mol������Ϊ�ȶ������
��2�����������ȷֽ������������ƺ�����������Ϊ��ȼ���壻
��3������ˮ��Ӧ�����������ƺ�����������Ϊ��ȼ���壻
��4��̼�������ǿ��HCN������ǿ���Ʊ������֪���軯���������̼��ˮ��Ӧ����HCN��̼�����ƣ�
��5���������ƺ��軯�Ʒ���������ԭ��Ӧ�����Ȼ��ơ�������̼��������̼���ƣ�
��� ��1��44g�������������ʵ���Ϊ$\frac{44g}{88g/mol}$=0.5mol����ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ1122.1kJ��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������Ӧ���Ȼ�ѧ����ʽ����ȼ����дΪ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol��
�ʴ�Ϊ��CH3COOCH2CH3��l��+5O2��g��=4CO2��g��+4H2O��l������H=-2244.2kJ/mol��
��2����ȼ�������ƣ�����ȼ���Ż�ʱ���ֽܷ����һ�������������ƣ���Ӧ�Ļ�ѧ����ʽ��2NaNO3=2NaNO2+O2����
�ʴ�Ϊ��2NaNO3=2NaNO2+O2����
��3������ˮ��Ӧ�����������ƺ���������ѧ����ʽ��2Na+2H2O=2NaOH+H2����
�ʴ�Ϊ��2Na+2H2O=2NaOH+H2����
��4���軯���������̼��ˮ��Ӧ����HCN��̼�����ƣ���ѧ����ʽ��NaCN+CO2+H2O=HCN+NaHCO3��
�ʴ�Ϊ��NaCN+CO2+H2O=HCN+NaHCO3��
��5���������ƺ��軯�Ʒ���������ԭ��Ӧ�����Ȼ��ơ�������̼��������̼���ƣ���ѧ����ʽ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3��
�ʴ�Ϊ��5NaClO+2NaCN=5NaCl+CO2��+N2��+Na2CO3��
���� ���⿼���˻�ѧ����ʽ����д����ȷ���ʵ����ʼ�������Ӧ��ʵ���ǽ���ؼ�����Ŀ�ѶȲ���

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| A�� | aΪ��Դ�ĸ��� | |
| B�� | ������Ӧ����ʽΪ��Ag-e-+Cl-�TAgCl | |
| C�� | �����ĵ缫��ӦʽΪ2HSO3-+2H++2e-�TS2O42-+2H2O | |
| D�� | ����·��ת�Ƶ��ӵ����ʵ���Ϊ5��10-5 molʱ�����봫������SO2Ϊ1.12 mL |
| A�� | ����մ�����ϣ�����������������Һϴ�� | |
| B�� | ������ʧ����������ĭ�������� | |
| C�� | �ƾ���ʧ���������ˮ���� | |
| D�� | �������ռ���Һ����Ƥ���ϣ�������ˮ��ϴ |
| A�� | 25��ʱ��NH4Cl��NH3��H2O�Ļ��Һ������Ũ�ȿ���ΪC��NH4+����C��Cl-����C��OH-����C��H+�� | |
| B�� | ��0.1mol/L��CuSO4��Һ�м�����������ˮ����Һ���Լ�����Cu2+ˮ��̶Ƚ��� | |
| C�� | 25��ʱ��PH=12��NaOH��Һ��PH=12��CH3COONa��Һ��PH=2�����ᣬ������Һ��ˮ�ĵ���̶���ͬ | |
| D�� | 25��ʱ����ϡ��ˮ��ͨ��NH3����Һ��C��OH-����С |



 R1COOH+R2COOH ��R1��R2����������
R1COOH+R2COOH ��R1��R2����������