��Ŀ����
����Ŀ��ʵ�����ﳣ��Ũ������������̷�Ӧ����ȡ��������������Ӧ�Ļ�ѧ����ʽΪ��MnO2��4HCl(Ũ) ![]() MnCl2��Cl2����2H2O��ȡһ������Ũ����ʹ����������̷�����Ӧ�������������ڱ�״���µ����Ϊ22.4 L��
MnCl2��Cl2����2H2O��ȡһ������Ũ����ʹ����������̷�����Ӧ�������������ڱ�״���µ����Ϊ22.4 L��
��ش��������⣺
(1)�μӷ�Ӧ�Ķ������̵�����Ϊ____________��
(2)��Ӧ�б�������HCl�����ʵ���Ϊ ____________��
(3)ʵ���ұ��õ�Ũ������������Ϊ36.5%���ܶ�Ϊ1.19g��cm��3����������Ũ��������ʵ���Ũ��______________��
���𰸡���1��87 g����2��2 mol����3��11.9 mol��L-1��
��������
���⣨1�����ݷ�Ӧ����ʽ���ó���n(MnO2)=n(Cl2)=22.4/22.4mol=1mol����m(MnO2)=1��87g=87g����2�������ڴ˷�Ӧ����ʽ�У�һ������ԭ����һ���������ԣ���˱�������HCl�����ʵ���=2n(Cl2)=2��22.4/22.4mol=2mol����3������c=1000��w%/M=1000��1.19��36.5%/36.5mol��L��1=11.9mol��L��1��

����Ŀ��Ԫ�����ڱ��е������ڰ���Na��Mg��Al��Si��P��S��Cl��Ar 8��Ԫ�ء���ش��������⣺
(1)��̬��ԭ�Ӻ�����________���˶�״̬��ͬ�ĵ��ӡ�
(2)��������8��Ԫ�ذ������۵�(��)��С˳����Ƶ�����ͼ(��֪���Ρ�1������Ar)������ʾ�������С�2��ԭ�ӵĽṹʾ��ͼΪ________����8�����ʾ��������Ϊ________��


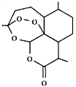
(3)�⻯þ
������ϵľ����ṹ��ͼ��ʾ����֪�þ�����ܶ�Ϊ�� g��cm��3����þ���Ļ�ѧʽΪ________�����������Ϊ________cm3(������NA��ʾ������NA��ʾ�����ӵ�������ֵ)��
(4)ʵ��֤����KCl��MgO��CaO���־���Ľṹ��NaCl����Ľṹ���ƣ���֪NaCl��KCl��CaO����ľ������������±���
���� | NaCl | KCl | CaO |
������/(kJ��mol��1) | 786 | 715 | 3 401 |
��KCl��MgO��CaO���־�����۵�Ӹߵ��͵�˳����________________������MgO������һ��Mg2����Χ��������ҵȾ����Mg2����________����
(5)Si��C��O�ijɼ�������£�
��ѧ�� | C-O | C=O | Si-O | Si=O |
����/(kJ��mol��1) | 360 | 803 | 464 | 640 |
C��O֮�����γɺ���˫����CO2���Ӿ��壬��Si��O֮�������γɺ��е�����SiO2ԭ�Ӿ��壬�������ݷ�����ԭ��__________________________________________��