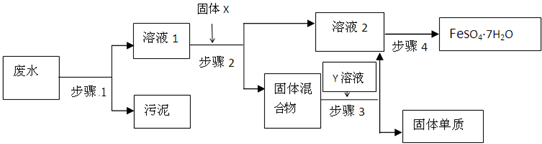
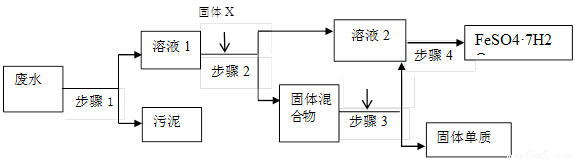
��Ŀ����
ʵ����Ҫ����100mL1.0mol?L-1��NaCl��Һ���Իش����и��⣺
��1�������㣬Ӧ����������ƽ��ȡNaCl����
��2������NaCl����������Һ�������ձ�����������ҩ�ס�������ƽ��ϴƿ���Ҫ�õ��IJ���������
��3������ƿ�ϳ��̶����Ӧ����
��4�����������������ᵼ��������ҺŨ��ƫ�ߵ���
A������ʱ��������ƿ�̶��� B������ʱ��������ƿ�̶���
C�����ܽ���ȴ�����Һת������ƿ�о�ֱ�ӽ��ж��ݲ���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ�
��1�������㣬Ӧ����������ƽ��ȡNaCl����
5.9
5.9
g����2������NaCl����������Һ�������ձ�����������ҩ�ס�������ƽ��ϴƿ���Ҫ�õ��IJ���������
100mL����ƿ
100mL����ƿ
����ͷ�ι�
��ͷ�ι�
����3������ƿ�ϳ��̶����Ӧ����
�¶�
�¶�
���ݻ�
�ݻ�
��ʹ������ƿǰ��һ��Ҫ��©
��©
����4�����������������ᵼ��������ҺŨ��ƫ�ߵ���
B
B
������ţ���A������ʱ��������ƿ�̶��� B������ʱ��������ƿ�̶���
C�����ܽ���ȴ�����Һת������ƿ�о�ֱ�ӽ��ж��ݲ���
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ�
��������1������n=cV������ʵ����ʵ������ٸ���m=nM������ʵ�������������ƽ�ľ�ȷ��ȷ��������������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3����������ƿ�ϱ����¶ȡ��������̶��ߣ�����ƿʹ��ǰ�����©��
��4������C=
���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߣ�
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3����������ƿ�ϱ����¶ȡ��������̶��ߣ�����ƿʹ��ǰ�����©��
��4������C=
| n |
| V |
����⣺��1��NaCl�����ʵ���n=cV=0.1L��1.0mol?L-1=0.1mol��NaCl������Ϊ0.1mol��58.5g/mol=5.85g������ƽ�ľ�ȷ��Ϊ0.1g������������ƽʵ�ʳ�ȡNaCl���������Ϊ5.9g���ʴ�Ϊ��5.9��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��3��������ƿ�ϱ����¶ȡ��������̶��ߣ�����ƿʹ��ǰ�����©���ʴ�Ϊ���¶ȡ��������̶��ߣ���©��
��4��A������ʱ��������ƿ�̶��ߣ���Һ��Һ�泬���̶��ߣ����ƫ��Ũ��ƫС����A����
B������ʱ��������ƿ�̶��ߣ���Һ��Һ����ڿ̶��ߣ����ƫС��Ũ��ƫ��B��ȷ��
C�����ܽ���ȴ�����Һת������ƿ�о�ֱ�ӽ��ж��ݲ�������δϴ�ӣ���ʹ���ʵ��������٣�Ũ��ƫС����C����
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ������ڿ̶������ϵ�Һ������������������䣬Ũ�Ȳ��䣬��D����
��ѡ��B��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��3��������ƿ�ϱ����¶ȡ��������̶��ߣ�����ƿʹ��ǰ�����©���ʴ�Ϊ���¶ȡ��������̶��ߣ���©��
��4��A������ʱ��������ƿ�̶��ߣ���Һ��Һ�泬���̶��ߣ����ƫ��Ũ��ƫС����A����
B������ʱ��������ƿ�̶��ߣ���Һ��Һ����ڿ̶��ߣ����ƫС��Ũ��ƫ��B��ȷ��
C�����ܽ���ȴ�����Һת������ƿ�о�ֱ�ӽ��ж��ݲ�������δϴ�ӣ���ʹ���ʵ��������٣�Ũ��ƫС����C����
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ������ڿ̶������ϵ�Һ������������������䣬Ũ�Ȳ��䣬��D����
��ѡ��B��
���������⿼����һ�����ʵ���Ũ����Һ�����ƹ��̡������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����

��ϰ��ϵ�д�
�����Ŀ