��Ŀ����
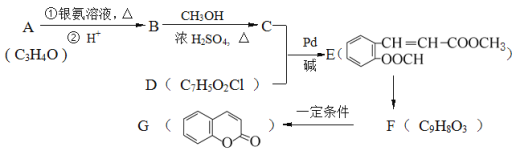
����Ŀ����ͼ��ʾΪijЩ�л���֮����ת����ϵ������A��B���ڷ����廯�����B������FeCl3��Һ������ɫ��Ӧ��H�Ǻ���һ������ʯ�ͻ�����չˮƽ��־�����ʡ�
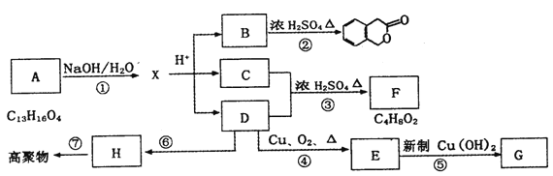
��ش��������⣺
��1��д�����л�����Ľṹ��ʽ��G____________��B____________��
��2��д�����з�Ӧ���ͣ���_____________����______________��
��3��д����Ӧ�١��ܵĻ�ѧ����ʽ����______________����____________��
��4����������3��������B��ͬ���칹�����Ŀ��________����
�ٺ����ڶ�ȡ�������ṹ����B����ͬ�����Ţ۲���FeCl3��Һ������ɫ��Ӧд����������һ��ͬ���칹��Ľṹ��ʽ_____________��
���𰸡�CH3COONa 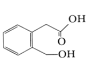 ˮ�� ��ȥ
ˮ�� ��ȥ ![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa 2CH3CH2OH��O2
+CH3CH2OH��CH3COONa 2CH3CH2OH��O2![]() 2CH3CHO��2H2O 3
2CH3CHO��2H2O 3 ![]() ��
��![]() ��
��
��������
��H�Ǻ���һ������ʯ�ͻ�����չˮƽ��־�����ʿ�֪��HΪCH2=CH2����D������������������Ӧ��֪��D���ڴ�����D������ȥ��Ӧ���ɵõ���ϩ����DΪCH3CH2OH���Ҵ�������������Ӧ������CH3CHO����EΪCH3CHO����ȩ������������ͭ������Һ�б�����Ϊ�����ƣ���GΪCH3COONa����ϩ�����Ӿ۷�Ӧ���ɸ߾���![]() ��C���Ҵ���Ũ���������·���������Ӧ����F����F�ķ���ʽ��֪��CΪCH3COOH��FΪCH3COOCH2CH3��A����ˮ�����ữ�����Ҵ��������B��A��B���ڷ����廯�����B����ʹFeCl3��Һ����ɫ��˵��B�������ǻ��������Ȼ������ǻ�����B�����γ���Ԫ���������A�ķ���ʽ��֪��BΪ
��C���Ҵ���Ũ���������·���������Ӧ����F����F�ķ���ʽ��֪��CΪCH3COOH��FΪCH3COOCH2CH3��A����ˮ�����ữ�����Ҵ��������B��A��B���ڷ����廯�����B����ʹFeCl3��Һ����ɫ��˵��B�������ǻ��������Ȼ������ǻ�����B�����γ���Ԫ���������A�ķ���ʽ��֪��BΪ ��AΪ
��AΪ![]() ��
��
��1���ɷ�����֪��G�Ľṹ��ʽΪCH3COONa��B�Ľṹ��ʽΪ ���ʴ�Ϊ��CH3COONa��
���ʴ�Ϊ��CH3COONa�� ��
��
��2����Ӧ����![]() ������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�
������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�![]() ����Ӧ�����Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ���ʴ�Ϊ��ˮ�ⷴӦ����ȥ��Ӧ��
����Ӧ�����Ҵ���Ũ���������£����ȵ�170�淢����ȥ��Ӧ������ϩ��ˮ���ʴ�Ϊ��ˮ�ⷴӦ����ȥ��Ӧ��
��3����Ӧ����![]() ������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�
������������Һ�з�����ˮ�ⷴӦ�����Ҵ��������ƺ�![]() ��Ӧ�Ļ�ѧ����ʽΪ
��Ӧ�Ļ�ѧ����ʽΪ![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa ����Ӧ��Ϊ��ͭ�����������£��Ҵ�������������Ӧ������CH3CHO����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2
+CH3CH2OH��CH3COONa ����Ӧ��Ϊ��ͭ�����������£��Ҵ�������������Ӧ������CH3CHO����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O ���ʴ�Ϊ��
2CH3CHO��2H2O ���ʴ�Ϊ��![]() ��2NaOH
��2NaOH![]() +CH3CH2OH��CH3COONa ��2CH3CH2OH��O2
+CH3CH2OH��CH3COONa ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O ��
2CH3CHO��2H2O ��
��4��BΪ ��B��ͬ���칹����Ϻ����ڶ�ȡ�������ṹ����B����ͬ�����š�����FeCl3��Һ������ɫ��Ӧ��˵�������в����з��ǻ������д��ǻ����Ȼ�������������Ľṹ��ʽΪ
��B��ͬ���칹����Ϻ����ڶ�ȡ�������ṹ����B����ͬ�����š�����FeCl3��Һ������ɫ��Ӧ��˵�������в����з��ǻ������д��ǻ����Ȼ�������������Ľṹ��ʽΪ![]() ��
��![]() ��
�� ����3�֣��ʴ�Ϊ��
����3�֣��ʴ�Ϊ��![]() ��
��![]() ��
�� ��
��

����Ŀ������ʵ������Լ�ʵ��������ȫһ�µ��ǣ� ��
A | ������ͭ��Һ�м���һС������� | �к�ɫ�������� |
B | ��̼������Һ��ͨ����� | �а�ɫ�������� |
C | �����Ƶ���ˮ�ε���ɫʯ����ֽ�� | ��ֽ��� |
D | ����ɰֽ��ĥ�����������ھƾ��ƻ����ϼ��� | ���ۻ����������� |
A.AB.BC.CD.D