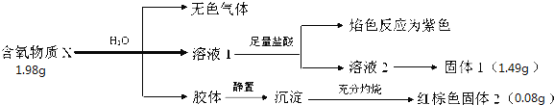
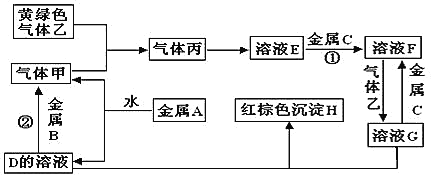
��Ŀ����
����Ŀ��ʵ����Ҫ����100mL1.0mol��L��1��NaCl��Һ���Իش����и��⡣
(1)�����㣬Ӧ����������ƽ��ȡNaCl����_________g��
(2)����NaCl����������Һ�����������У�����Ҫ�õ�����_________(�����)��
A.��ƿ��B.200mL����ƿ��C.�ձ���D.��ͷ�ιܣ�E.ҩ�ף�F.������ƽ��G.ϴƿ
(3)��Ҫʵʩ���ƣ������������⣬��ȱ����������Ʒ��_________��_________��
(4)��ʹ��ǰ����������ƿ�Ƿ�______��
(5)���ƹ��������²�����A.��Һ B.���� C.ϴ�� D.���� E.�ܽ� F.ҡ�ȡ�����ȷ�IJ���˳��Ӧ��_________ (�����)��
(6)���������������ᵼ��������ҺŨ��ƫ�ߵ���______(�����)��
A.����ʱ��������ƿ�̶���
B.����ʱ��������ƿ�̶���
C.���ܽ���ȴ����Һת������ƿ���ֱ��ת�붨�ݲ���
D.���ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
(7)��ʵ������г������������δ�����������ˮʱ���������˿̶ȣ�______��
���𰸡�5.9 AB ������ 100mL����ƿ ©ˮ B��E��A��C����A����D��F B Ӧ������Һ��ϴ������ƿ����������
��������
(1)����n=cVM��������100mL 1.0molL-1��NaCl��Һ��Ҫ�Ȼ���������
(2)����ʵ������IJ���(���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���)ѡ����Ҫ�����������жϲ���Ҫ��������
(3)����ʵ������IJ���(���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���)ѡ����Ҫ�����������ж�ȱ��������
(4)��������ƿ�Ľṹ��ʹ�÷���������
(5)����ʵ������IJ���(���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ���)��������
(6)�����������������ʵ�������Һ�����Ӱ�죬����c=![]() ����������������ҺŨ�ȵ�Ӱ�죻
����������������ҺŨ�ȵ�Ӱ�죻
(7)������ˮʱ���������˿̶ȣ������ȣ�Ӧ������Һ��ϴ������ƿ���������ơ�
(1)����100mL1.0molL-1��NaCl��Һ��Ҫ�Ȼ�������Ϊ1.0molL-1��0.1L��58.5g/mol=5.9g��
(2)ʵ������IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬���ò��������裬�ָ����º�ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һ��������ƿ�У���������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ����Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�����ҡ�ȣ������Լ�ƿ����ǩ���棻������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ�������ϴƿ��ˮ���ʲ���Ҫ�õ��������У�A����ƿ��B��200mL����ƿ����ѡ��AB��
(3)��(2)�еIJ��������֪��������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʻ�ȱ�ٵ�����Ϊ����������100mL����ƿ��
(4)����ƿƿ���в�������ʹ��ʱ��Ҫ�ߵ�ҡ�ȣ�����ʹ��ǰ����������ƿ�Ƿ�©ˮ��
(5)��(2)�еIJ��������֪����ȷ�IJ���˳��ΪB��E��A��C��(A)��D��F��
(6)A������ʱ��������ƿ�̶��ߣ�����������Һ���ƫ��������Һ��Ũ��ƫ�ͣ���A�����ϣ�
B������ʱ��������ƿ�̶��ߣ�����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ���B���ϣ�
C�����ܽ���ȴ����Һ��ת������ƿ���ֱ��ת�붨�ݲ������ձ��Ͳ������в���������û��ת��������ƿ�������ܶ�ƫ�ͣ���C�����ϣ�
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬���伸��ˮ���̶ȴ���ʹ������Һ�����ƫ��������ҺŨ��ƫ�ͣ���D�����ϣ�
�ʴ�ΪB��
(7)������ˮʱ���������˿̶ȣ���Һ�����ƫ��������ҺŨ��ƫ�ͣ������ȣ�Ӧ������Һ��ϴ������ƿ���������ơ�

����Ŀ����20 L���ܱ������а����ʵ���֮��Ϊ1��2����CO��H2��������Ӧ��CO(g)��2H2(g)![]() CH3OH(g)����H�����CO��ת�������¶ȼ���ͬѹǿ�µı仯��ͼ��ʾ��p2��195 ��ʱn(H2)��ʱ��ı仯��������ʾ������˵����ȷ����(����)
CH3OH(g)����H�����CO��ת�������¶ȼ���ͬѹǿ�µı仯��ͼ��ʾ��p2��195 ��ʱn(H2)��ʱ��ı仯��������ʾ������˵����ȷ����(����)
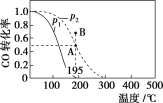
p2��195 �� ʱn(H2)��ʱ��仯
t/min | 0 | 1 | 3 | 5 |
n(H2)/mol | 8 | 5 | 4 | 4 |
A. p1��p2����H��0
B. ��p2��195 �� ʱ����Ӧǰ3 min��ƽ������v(CH3OH)��0.8 mol��L��1��min��1
C. ��p2��195 �� ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ25
D. ��B ��ʱ��v����v��