��Ŀ����
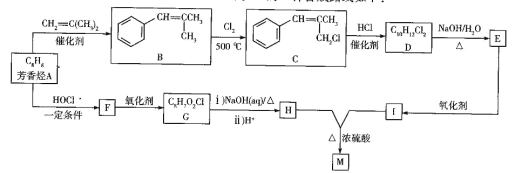
����Ŀ��TiO2��TiCl4��Ϊ��Ҫ�Ļ���ԭ�ϣ���֪��
��.TiCl4(g)+O2(g)![]() TiO2(s)+2Cl2(g) ��H=-175.4kJ/mol
TiO2(s)+2Cl2(g) ��H=-175.4kJ/mol
��.2C(s)+O2(g)![]() 2CO(g) ��H=-220.9kJ/mol
2CO(g) ��H=-220.9kJ/mol
��ش��������⣺
��1��TiCl4(g)+2CO(g)![]() TiO2(s)+2C(s)+2Cl2(g)����H=___kJ/mol��
TiO2(s)+2C(s)+2Cl2(g)����H=___kJ/mol��
��2��t��ʱ����10L�����ܱ������г���1molTiCl4��2molO2��������ӦI��4min�ﵽƽ��ʱ���TiO2�����ʵ���Ϊ0.2mol��
�ٷ�Ӧ0~4minĩ��ƽ������v(Cl2)=_�����¶���K=_���÷�����ʾ����O2��ƽ��ת����=__��
�����д�ʩ�����ܼӿ�����Ӧ���ʣ���������O2��ƽ��ת���ʵ���_��
A.�����������
B.�������
C.���������TiO2
D.����O2��Ũ��
E.�����¶�
F.���Ϸ�����������
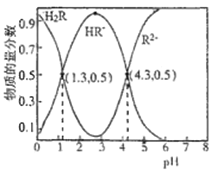
��t��ʱ����10L�����ܱ������г���3molTiCl4��һ������O2��������ӦI�����������ƽ��ת���ʣ�a%������ʼ�����ʵ���֮�ȣ�TiCl4/O2���Ĺ�ϵ��ͼ1��ʾ���ܱ�ʾO2��ƽ��ת���ʵ�����Ϊ___(����L1������L2��)��M�������Ϊ___(�������������ʾ)��
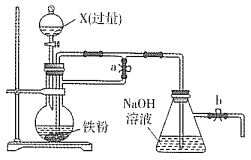
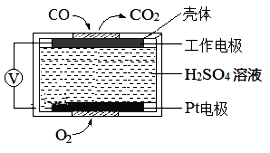
��3��CO���ж����壬�ⶨ������CO�������õķ���֮һ�ǵ绯ѧ������������������CO�������Ĺ���ԭ����ͼ2��ʾ�������缫�ķ�ӦʽΪ___��Ϊ���ٶԻ�����ɵ�Ӱ�죬��CO��H2�����Ʊ��״�(CH3OH)���Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ�أ��缫����Ϊ���Ե缫�����������Һ��KOH�����ʵ���Ϊ0.8mol������0.5mol�״����뷴Ӧʱ���������Һ�����ʵ���Ҫ�ɷ���___(д��ѧʽ)��
���𰸡�+45.5 0.01mol/(Lmin) ![]() 10% F L1 (1��
10% F L1 (1��![]() ) CO��2e-��H2O=CO2��2H+ K2CO3��KHCO3
) CO��2e-��H2O=CO2��2H+ K2CO3��KHCO3
��������
(1)��֪��. TiCl4(g)+O2(g)![]() TiO2(s)+2Cl2(g) ��H=-175.4kJ/mol����.2C(s)+O2(g)
TiO2(s)+2Cl2(g) ��H=-175.4kJ/mol����.2C(s)+O2(g)![]() 2CO(g) ��H=-220.9kJ/mol������-���ɵ�TiCl4(g)+2CO(g)
2CO(g) ��H=-220.9kJ/mol������-���ɵ�TiCl4(g)+2CO(g)![]() TiO2(s)+2C(s)+2Cl2(g)�����ݸ�˹���ɿɵã���H=-175.4kJ/mol-(-220.9kJ/mol)=+45.5kJ/mol���ʴ�Ϊ��+45.5��
TiO2(s)+2C(s)+2Cl2(g)�����ݸ�˹���ɿɵã���H=-175.4kJ/mol-(-220.9kJ/mol)=+45.5kJ/mol���ʴ�Ϊ��+45.5��
(2)���������Ϣ��֪��
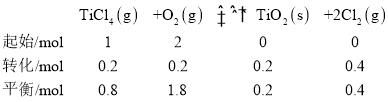
��0~4min�ڣ���Cl2��ʾ�ķ�Ӧ����![]() ��ƽ�ⳣ��
��ƽ�ⳣ��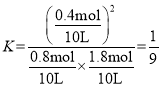 ��
��![]() ���ʴ�Ϊ��0.01mol/(Lmin)��
���ʴ�Ϊ��0.01mol/(Lmin)��![]() ��10%��
��10%��
��A. ��С�������������ѹǿ��������Ӧ���ʼӿ죬��ƽ�ⲻ�ƶ���O2��ƽ��ת���ʲ��䣬Aѡ�����
B���������������Ӧ���ʼӿ죬��ƽ�ⲻ�ƶ���O2��ƽ��ת���ʲ��䣬Bѡ�����
C�����������TiO2������Ӧ���ʼ�����ƽ�������ƶ���O2��ƽ��ת��������Cѡ�����
D������O2��Ũ�ȣ�����Ӧ���ʼӿ죬ƽ�������ƶ�����������������ԭ����ֻ���������ܵ�����O2��ƽ��ת���ʽ��ͣ�Dѡ�����
E�������¶ȣ�����Ӧ���ʼ�����ƽ�������ƶ���O2��ƽ��ת��������Eѡ�����
���ϣ����Ϸ�������������������F��ȷ����Ϊ��F��
������TiCl4��Ũ�����ӣ�O2��ת����Խ��Խ������L1��ʾO2��ƽ��ת���ʵ����ߣ�M���ʾn(TiCl4/O2)=����ʽ������֮��=1ʱ��TiCl4��ת���ʺ�O2��ת������ͬ����a=1��t��ʱ����10L�����ܱ������г���3molTiCl4��3molO2�Ļ������ʱ��ת������ȣ���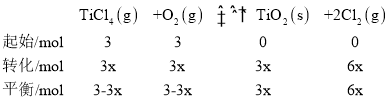
��Ϊ�¶Ȳ��䣬��ƽ�ⳣ�����䣬�� �����x=
�����x=![]() ��M���������
��M�������Ϊ��![]() ���ʴ�Ϊ��L1��
���ʴ�Ϊ��L1��![]() ��
��
(3)��ͼ��֪��H2SO4���������Һ�������缫��COʧȥ����ת��ΪCO2����缫��ӦʽΪCO��2e-��H2O=CO2��2H+���Լ״�Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ�أ��缫����Ϊ���Ե缫�����״��ڸ������뷴Ӧ������Ԫ���غ㣬����/span>0.5mol�״����뷴Ӧʱ��������CO2��0.5mol����0.8mol�������ط�Ӧ�����ݷ���ʽ2KOH+CO2===K2CO3+H2O��K2CO3+CO2+H2O===2KHCO3����֪����0.3mol��K2CO3��0.2mol��KHCO3����������Һ�����ʵ���Ҫ�ɷ���K2CO3��KHCO3���ʴ�Ϊ��CO-2e-+H2O===CO2+2H+��K2CO3��KHCO3��

����Ŀ��SO2��һ�ִ�����Ⱦ������ڻ�����ʳƷ��ҵ��ȴ�й㷺Ӧ�á�ij��ȤС��ͬѧ��SO2��ʵ�����Ʊ�������ʵ������о���
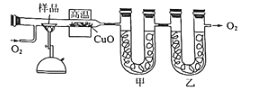
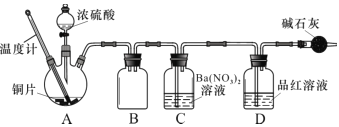
(1)��ͬѧ���ս̲�ʵ��Ҫ�������ͼ��ʾװ����ȡSO2

�ٱ�ʵ����ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ______��ͭ˿�ɳ鶯���ŵ���_______��
��ʵ�������ͬѧ�۲쵽�Թܵײ����ֺ�ɫ�ͻҰ�ɫ���壬����Һ��ɫ���ڡ���ͬѧ��Ϊ�Ұ�ɫ����Ӧ�����ɵİ�ɫCuSO4����������ɫ����Ļ�������CuSO4��ɫ������ʽ����������Ũ�����________�ԡ�
����ͬѧ��Ϊ��ʵ����ƴ������⣬���ʵ�鰲ȫ�ͻ����Ƕȷ�������ʵ���п��ܴ��ڵ�������________��
(2)��ȤС�����������ϣ������ۺϷ������ۣ��������ʵ������(����װ����)��
ʵ���¼ A ���������£�
��� | ��Ӧ�¶�/�� | ʵ������ |
1 | 134 | ��ʼ���ֺ�ɫ��״��������³������������ |
2 | 158 | ��ɫ���������ͬʱ������������ |
3 | 180 | ����ų����ٶȸ��죬�Թ�����ҺΪ��ɫ���� |
4 | 260 | �д��������������Һ��Ϊ��ɫ���Թܵײ������Ұ�ɫ���壬Ʒ����Һ��ɫ |
5 | 300 | ͬ�� |
�������ϵ�֪�� �����еĺ�ɫ�ͻҰ�ɫ����������Ҫ�ɷ�Ϊ CuS��Cu2S �� CuSO4������CuS �� Cu2SΪ��ɫ���壬�����¶�������ϡ���ᣬ�ڿ��������վ�ת��ΪCuO��SO2��
��ʵ����ʢװŨ�������������Ϊ ____________��
��ʵ���¼����__________��ʵ������Ӱ�죬Ϊ�˵õ�Ԥ��ʵ�������ڲ�����Ӧ��____________��
��װ��C �з�����Ӧ�����ӷ���ʽ�� ___________________��
�ܽ�ˮϴ������ĺ�ɫ�����ɺⶨ����ǰ��������仯�����Խ�һ��ȷ����ɫ�������Ƿ�һ������ CuS��ԭ��Ϊ__________(��ϻ�ѧ����ʽ����)��