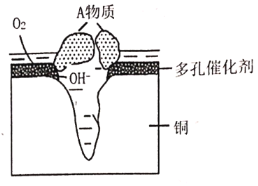
��Ŀ����
����Ŀ���ݲ���ȫͳ�ƣ�ȫ����ÿ��Լ��10%��20%��ũ����Ʒ��ˮ��Ʒ���ܱ��ʶ���ʳ�ã�������ʧ�������о���ȫ����ʳƷ����������Ҫ��ɽ���ἰ���Σ��Ჴ�����ȿ���ΪʳƷ����������ṹ��ʽ��ͼ��
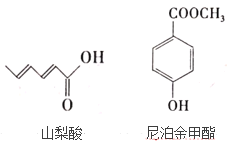
��Ҫ��ش��������⣺
��1��д��ɽ�����к��������ŵ�����___��
��2�������£������ʵ�����ɽ���ᡢ�Ჴ������ֱ���������ˮ��Ӧ������Br2�����ʵ���֮��Ϊ___��
��3��д���Ჴ�������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ____��
���𰸡��Ȼ� 1:1 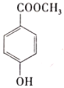 +2NaOH
+2NaOH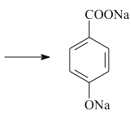 +CH3OH+H2O
+CH3OH+H2O
��������
��1��ɽ�����еĺ���������Ϊ�Ȼ���
��2�����ݶ���������ˮ��Ӧ�Ĺ������жϣ�
��3���Ჴ������к��������ͷ��ǻ�����������������Һ��Ӧ��
��1������ɽ����Ľṹ��ʽ��ɽ�����еĺ���������Ϊ�Ȼ���
��2�����ݶ���������ˮ��Ӧ�Ĺ���������ȷ���ܺͶ���mol�巴Ӧ��ɽ�����к���2mol̼̼˫��������2molBr2�����ӳɷ�Ӧ���Ჴ������к��з��ǻ�����������λ����ˮ����ȡ����Ӧ�������Ჴ���������2molBr2������Ӧ������Br2�����ʵ���֮��Ϊ1��1��
��3���Ჴ������к��������ͷ��ǻ����������������Ʒ�Ӧ������ʽΪ +2NaOH
+2NaOH +CH3OH+H2O��
+CH3OH+H2O��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�����Ŀ�����ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� |
��1���ܡ���Ԫ�ط��ŷֱ���_____��______
��2����Ԫ���⻯��ĵ���ʽ��________
��3����Ԫ�ص�ԭ�ӽṹʾ��ͼ��__________
��4��Ԫ�آ٣�����ԭ�Ӱ뾶������_____��
��6����ʢ��ˮ��С�ձ��м���Ԫ�آݵĵ��ʣ�������Ӧ�����ӷ���ʽΪ______����������Ӧ�����Һ�м���Ԫ�آĵ��ʣ�������Ӧ�Ļ�ѧ����ʽΪ______��