��Ŀ����
4����Ԫ�غ�����26��������1����Ԫ��λ��Ԫ�����ڱ��е������ڣ��ڢ��壮
��2��Fe3+�ĵ����Ų�ʽ1s22s22p63s23p63d5��
��3��Fe2+����Χ���ӹ����ʾʽ
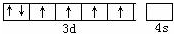 ��
����4��ΪʲôFe3+����Ȼ��Fe�ĸ��ִ�����ʽ����Ϊ������Fe3+�ĺ�������Ų�Ϊ1s22s22p63s23p63d5��d���������Ϊ�ȶ�״̬��
��5��Ŀǰ���ֵ���������ϼ���+6�ۣ������������+8��
���� ��1�������������ڱ��е�λ�÷����ش�
��2����Ԫ�ط��ţ��ж����ĺ�����������ٸ��ݺ�������Ų�������д��
��3��Fe2+����Χ����Ϊ3d6���ݴ���д�����ʾʽ��
��4����Χ���������ȫ����Ϊ�ȶ�״̬��
��5����Χ��������������ϼۣ�
��� �⣺��1����λ�����ڱ��еĵ������ڣ��ڢ��壬���ڹ���Ԫ�أ��ʴ�Ϊ���ģ�����
��2����ԭ��ʧȥ�����4s�ܼ�2�����ӣ�Ȼ��ʧȥ3d�ܼ��ϵ�1�������γ�Fe3+����������Ų�ʽΪ��1s22s22p63s23p63d5��
�ʴ�Ϊ��1s22s22p63s23p63d5��
��3��Fe2����Χ����Ϊ3d6��������ʾʽ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��Fe2+�ĺ�������Ų�Ϊ1s22s22p63s23p63d6��Fe3+�ĺ�������Ų�Ϊ1s22s22p63s23p63d5��d���������Ϊ�ȶ�״̬��
�ʴ�Ϊ��Fe3+�ĺ�������Ų�Ϊ1s22s22p63s23p63d5��d���������Ϊ�ȶ�״̬��
��5��������Χ������Ϊ8������������������ϼ�Ϊ+8�ۣ��ʴ�Ϊ��+8��
���� ���⿼��������λ�����ʣ�ԭ�ӽṹ�����պ�������Ų������ǹؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
12��ij������Һ���ܶ�Ϊ1.48g/cm3��������������Ϊ49%������Һ�����ʵ����ʵ���Ũ���ǣ�������
| A�� | 0.0074mol/L | B�� | 0.74mol/L | C�� | 7.4mol/L | D�� | 4.9mol/L |
9��ijϡ��Һ����Fe2+��Na+��Al3+��Ba2+��SO42-��NO3-��Cl-�е�4�����ӣ��������ӵ����ʵ�����Ϊ1mol���������Һ�м���������ϡ���ᣬ�����ݲ���������Һ������������䣨������ˮ�ĵ�������ӵ�ˮ�⣩������˵����ȷ���ǣ�������
| A�� | �������Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù��������Ϊ72g | |
| B�� | �������Һ�м��������ϡ���ᣬ��Һ��ɫ����dz��ɫ | |
| C�� | �������Һ�м��������ϡ�����KSCN��Һ����Һ����Ѫ��ɫ | |
| D�� | �������Һ�м��������ϡ���ᣬ�����������ڱ���µ����ԼΪ7.467 L |
16�� ѧϰС���������ͼʵ�飨����װ�ã�������������ͨ������װ������֤���������ʣ�
ѧϰС���������ͼʵ�飨����װ�ã�������������ͨ������װ������֤���������ʣ�
��1��ͨ��������A�е���������Һ����ɫ��Cװ���з�����Ӧ�����ӷ���ʽΪ��2Fe2++Cl2�T2Fe3++2Cl-��
��2��ͨ������һ��ʱ���ϴ��ƿB��Һ����һ����SO32-������SO42-��������鷽��������ϴ��ƿB��Һ��Cl-��SO42-�Ĵ��ڣ��ڴ���ϲ�����д��ʵ�鲽�衢Ԥ������ͽ��ۣ�
��ѡ�Լ���������2mol/LHCl��2mol/LHNO3��1mol/LBaCl2��Һ��lmol/LBa��NO3��2��Һ��0.1mol/LAgNO3��Һ������ʯ��ˮ���Թܡ���ͷ�ιܣ�
��3��Ϊȷ�ⶨͨ������һ��ʱ���ʣ��FeCl2�����ʵ�����ʵ�����£�
������250mL ��Һ��ȡϴ��ƿC��ȫ����Һȷ����250mL��Һ��
�ڵζ���ȷ��ȡ25.00ml������Һ����ƿ�У���0.1980mol/LKMnO4��Һװ����ʽ�ζ��ܣ��ζ����յ㣬��¼���ݣ��ظ��ζ�2�Σ�ƽ������KMnO4��ҺV mL������Ӧʽ��Fe2++MnO4-+H+-Fe3++Mn2++H2O��δ��ƽ��
�ۼ���250mL��Һ��FeCl2�����ʵ���=$\frac{250}{25.00}$��0.1980��V��10-3��5��10��0.1980��V��10-3��5mol��ֻ�г���ʽ���������㣩��
 ѧϰС���������ͼʵ�飨����װ�ã�������������ͨ������װ������֤���������ʣ�
ѧϰС���������ͼʵ�飨����װ�ã�������������ͨ������װ������֤���������ʣ���1��ͨ��������A�е���������Һ����ɫ��Cװ���з�����Ӧ�����ӷ���ʽΪ��2Fe2++Cl2�T2Fe3++2Cl-��
��2��ͨ������һ��ʱ���ϴ��ƿB��Һ����һ����SO32-������SO42-��������鷽��������ϴ��ƿB��Һ��Cl-��SO42-�Ĵ��ڣ��ڴ���ϲ�����д��ʵ�鲽�衢Ԥ������ͽ��ۣ�
��ѡ�Լ���������2mol/LHCl��2mol/LHNO3��1mol/LBaCl2��Һ��lmol/LBa��NO3��2��Һ��0.1mol/LAgNO3��Һ������ʯ��ˮ���Թܡ���ͷ�ιܣ�
| ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ����ϴ��ƿB����Һ���Թ�A�У��μӵμӹ���2mol/LHCl��1�֣���1mol/LBaCl2��Һ���� | �������İ�ɫ��������ϴ��ƿB��Һ�д���SO42-�� |
����2����ȡ����ϴ��ƿB����Һ���Թ�B�У��μӵμ������lmol/LBa��NO3��2��Һ�������ã� | ������ɫ������ |
����3��ȡ����2���Թ�B�е��ϲ���Һ���Թ�C�У� �μ�0.1mol/LAgNO3��Һ������2mol/LHNO3���� | ��������ɫ��������ϴ��ƿB��Һ�д��������ӣ� |
������250mL ��Һ��ȡϴ��ƿC��ȫ����Һȷ����250mL��Һ��
�ڵζ���ȷ��ȡ25.00ml������Һ����ƿ�У���0.1980mol/LKMnO4��Һװ����ʽ�ζ��ܣ��ζ����յ㣬��¼���ݣ��ظ��ζ�2�Σ�ƽ������KMnO4��ҺV mL������Ӧʽ��Fe2++MnO4-+H+-Fe3++Mn2++H2O��δ��ƽ��
�ۼ���250mL��Һ��FeCl2�����ʵ���=$\frac{250}{25.00}$��0.1980��V��10-3��5��10��0.1980��V��10-3��5mol��ֻ�г���ʽ���������㣩��
13����ʵ���ҿ�����Ũ������������̹������������Ʊ�װ�����밲װ��Һ©��������ʹ�ó���©�����й�ԭ������������ǣ�������
| A�� | ��ֹ����ͨ��©����ɢ�������������Ⱦ | |
| B�� | ���ڿ��Ƽ���Ũ������� | |
| C�� | ��©������ʱ�������� | |
| D�� | ��������HC1�ӷ��������� |
14������˵���д�����ǣ�������
| A�� | ���������Ƿǵ���� | |
| B�� | ������Ũ����ͬ������ʹ��ᵼ��������ͬ | |
| C�� | �������������ܵ��磬���������������ǵ���� | |
| D�� | ����ͬ�¶��£�������Һ��һ���Ȳ�������Һ�ĵ�����ǿ |
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺