��Ŀ����
����Ŀ��(14��)ijʵ��С����0.50 mol��L- 1 NaOH��Һ��0.50mol��L- 1 ��������Һ�����к��ȵIJⶨ��
 ��������0.50mol��L- 1 ������Һ
��������0.50mol��L- 1 ������Һ
��1��������250 mL������Һ����������Ͳ��ȡ�ܶ�Ϊ1.84 g��cm- 3����������Ϊ98����Ũ���� mL��
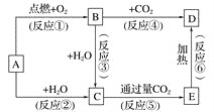
�����ⶨϡ�����ϡ����������Һ�к��ȵ�ʵ��װ������ͼ��ʾ��
��2������A������Ϊ ��
��3��װ��������ĭ���ϵ������� ��
��4��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��(�к���Ϊ57.3 kJ��mol- 1) ��
��5��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
���±��е��¶Ȳ�ƽ��ֵΪ ����
��������Ϊ0.50mol��L- 1 NaOH��Һ��0.50mol��L- 1 ������Һ���ܶȶ���1g��cm- 3 ���кͺ�������Һ�ı�����c=" 4.18" J��(g����)- 1 �����к�����H= (ȡС�����һλ)��

������ʵ����ֵ�����57.3 kJ��mol- 1 ��ƫ�������ʵ��ƫ���ԭ������ǣ�����ĸ�� ��
A��ʵ��װ�ñ��¡�����Ч���� |
B����ȡNaOH��Һ�����ʱ���Ӷ��� |
C��һ����NaOH��Һ����ʢ�������С�ձ��� |
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶� |
���𰸡�������1��6.8��������2�����β������������3�����¡����ȣ�������������ʧ����4��1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l)��H=-57.3KJ/mol��
��5����4.0����-53. 5KJ/mol����A��D��
������������������1����ϡ��ǰ�����ʵ����ʵ������䡣(0.50mol/L��0.25L)��98g/mol="1.84" g��cm- 3��V��98�������V=6.8ml����2������A������Ϊ���β������������3��װ��������ĭ���ϵ������DZ��¡����ȣ�������������ʧ,�Dzⶨ���¶ȸ�ȷ����4�������к����ǿ�����ǿ���������ǿ���ϡ��Һ������Ӧ�����������κ�1mol��ˮʱ���ų�����������˸÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��1/2H2SO4(aq)+NaOH(aq) =1/2Na2SO4(aq)+ H2O(l)��H=-57.3KJ/mol����5�������ᡢ�����ʵ���Ũ����ͬ�������Ƕ�Ԫ�ᣬ����ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����������Ӧ�ų����������ռ���м��㡣 ��ͨ���Ա��и������ݽ��й۲�ᷢ�֣���һ������ƫ��̫��Ӧ����ȥ����˱��е��¶Ȳ�ƽ��ֵΪ[��31.2��27.2������29.8��25.9������30.4��26.4��]��3="4.0��;" ��������Ϊ0.50mol��L- 1 NaOH��Һ��0.50mol��L- 1 ������Һ���ܶȶ���1g��cm- 3 ���кͺ�������Һ�ı�����c=" 4.18" J��(g����)- 1 �����к�����H=��cmt��n=(4.18��10-3��80��4.0)KJ��0.025mol="-53." 5KJ/mol. ������ʵ����ֵ�����57.3 kJ��mol- 1 ��ƫ�������ʵ��ƫ���ԭ�������A��ʵ��װ�ñ��¡�����Ч����,����ɢʧ��ʹ���ƫ�ͣ���ȷ�� B����ȡNaOH��Һ�����ʱ���Ӷ�����������ʵ���ƫ��������ƫ�࣬ʹ���ƫ�ߣ����� C��һ����NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�٣����С������ D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�ʹ�ڲⶨǰ�Ѿ��ɲ��ַ�����Ӧ���������ⶨֵƫ�٣���ȷ����ѡ����AD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��

��2��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ |
B������ƿ��ˮϴ�������ô�����Һϴ�� |
C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
D��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��3�����ݼ�����������ƽ��ȡ������Ϊ______g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��_____0.1mol/L��������������С������������������
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ_______mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��______mL��Ͳ��á�