��Ŀ����
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��
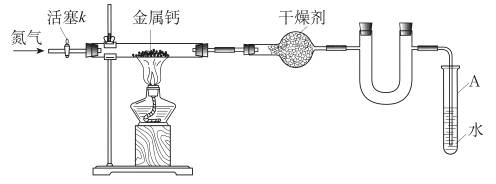
��1��д��ʵ�����Ʊ����������Ļ�ѧ����ʽ ��
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ�II�ٲ��л���ĺ�ȣ�ʵ���¼���£�
�ĺ�ȣ�ʵ���¼����
��1������ܵ������ǣ� ���Թܢ���ʢ�ŵ�̼������Һ ����ܡ����ܡ�����Ϊ����������Һ��ԭ���� ��
��2��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
��3������ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
��4������������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ���� ��
��1��д��ʵ�����Ʊ����������Ļ�ѧ����ʽ ��
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ�II�ٲ��л���ĺ�ȣ�ʵ���¼���£�

�ĺ�ȣ�ʵ���¼����
| ʵ���� | �Թܢ��е��Լ� | �Թܢ��е��Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2 mL���ᡢ1 mL 18mol/LŨ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6 mL 3mol/L���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |
��2��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
��3������ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
��4������������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ���� ��

��

��ϰ��ϵ�д�
�����Ŀ
 ��
�� ��
��
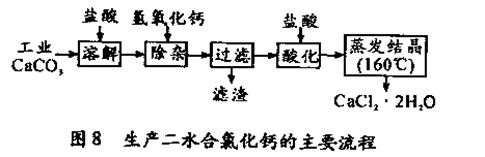
 �������Ͽ�֪���Ҷ��ᾧ��(H2C2O4��2H2O)�۵�100��1 �棬�������������ˮ�İ�ɫ���壻Cu2O������ϡ���ᣬ���������绯��Ӧ����Cu2+��Cu��
�������Ͽ�֪���Ҷ��ᾧ��(H2C2O4��2H2O)�۵�100��1 �棬�������������ˮ�İ�ɫ���壻Cu2O������ϡ���ᣬ���������绯��Ӧ����Cu2+��Cu�� ��һ��̽����
��һ��̽����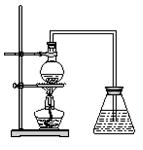


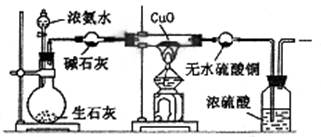
 2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O
2CuO,CuO+2HNO3="=" Cu(NO3)2 + H2O