��Ŀ����
ij��ѧ��ȤС������ij����������ͭп����ȡ����ZnOʵ���������£�
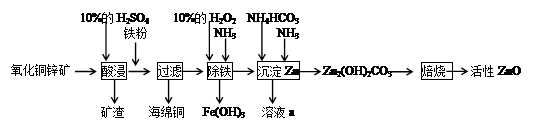
��ش��������⣺
��1���������ۺ�����Ӧ�����ӷ���ʽΪ__________��
��2���ס�����ͬѧѡ���������������ò�ͬ�ķ�������ȡ������
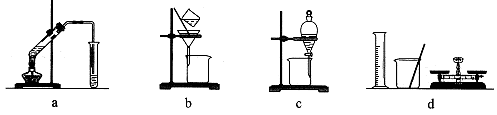
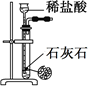
�ټ�ͬѧʹ�õ�ҩƷ����ʯ�����Ȼ�泥���Ӧѡ��װ��______����дװ�ô��ţ������ɰ����Ļ�ѧ����ʽΪ____________��
����ͬѧѡ����װ��B����ʹ�õ�����ҩƷ������Ϊ_______________��
��3��H2O2��������_____________��
��4�����������еõ���Fe(OH)3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ��������K2FeO4�����÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ____________��
��5����֪��Һa�к���CO32-��SO42-������������ӣ���ֻ����ȡ��һ����Ʒ�������������Ӵ��ڵ�ʵ���������Ϊ___________________��
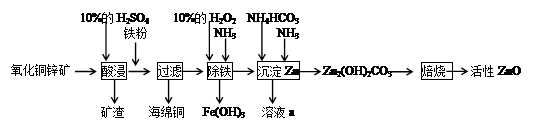
��ش��������⣺
��1���������ۺ�����Ӧ�����ӷ���ʽΪ__________��
��2���ס�����ͬѧѡ���������������ò�ͬ�ķ�������ȡ������

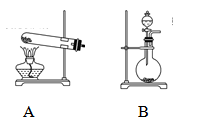
�ټ�ͬѧʹ�õ�ҩƷ����ʯ�����Ȼ�泥���Ӧѡ��װ��______����дװ�ô��ţ������ɰ����Ļ�ѧ����ʽΪ____________��
����ͬѧѡ����װ��B����ʹ�õ�����ҩƷ������Ϊ_______________��
��3��H2O2��������_____________��
��4�����������еõ���Fe(OH)3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ��������K2FeO4�����÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ____________��
��5����֪��Һa�к���CO32-��SO42-������������ӣ���ֻ����ȡ��һ����Ʒ�������������Ӵ��ڵ�ʵ���������Ϊ___________________��
��1��Fe+Cu2+= Fe2++Cu
��2��A Ũ��ˮ�ͼ�ʯ�ң�����ʯ�һ��������ƹ��壩
��3����Fe2+������Fe3+
��4��3��2
��5��ȡ������Һa���Թ��У��μ��Թ��������ᣬ����ɫ���ݲ�������˵����CO32-�������μ��Ȼ�����Һ��������ɫ������˵����SO42-
����������ZnO����һ������ϡ�������ܣ��������ܽ�����Zn2+��Cu2+���ڶ������뻹ԭ�����ۺ��ˣ�Cu2+����ԭ������ͬʱ��Һ��������Fe2+��������������ɫ������H2O2����Fe2+������Fe3+��ͨ�백ˮ������Һ��pH��ʹFe3+ת��ΪFe��OH��3�����IJ����ٴ�ͨ�백��������NH4HCO3��ʹ��Һ��Zn2+���ɼ�ʽ̼��п�����ˣ����գ��Ϳ��Եõ�����ZnO�������������������⣬�ǽ����Ĺؼ����ڣ�4����Ҫ���ü�̬�غ�ȥ�����������뻹ԭ�������ʵ���֮�ȵ�������1mol�õ���ʧȥ���ӵķ��ȣ����ڵڣ�5���ʣ�Ҫע����ǡ�һ�Ρ��ĺ��⣬��ȷѡ���Լ�����Ƽ���IJ��裬������п����ԣ�ֻҪ�������С�

��ϰ��ϵ�д�
�����Ŀ
 MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��

 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4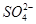 ��2H2O
��2H2O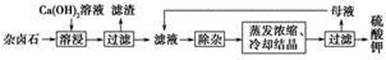
 ?
? CaCO3(s)��
CaCO3(s)��