��Ŀ����
ij���ķֽ����Ϊ̼�⻯���������̼�⻯����������ʵ�飺����ȡһ������ȫȼ�գ�ʹȼ�պ������ͨ������ܣ����������0.72g����ͨ��ʯ��ˮ��ʯ��ˮ����2.2g���ھ��ⶨ����̼�⻯������壩���ܶ�����ͬ�����������ܶȵ�34�����۸�̼�⻯����0.1mol�ܺ�32g����ӳɷ�Ӧ���ܾ��������ڢ۵��������У���ԭ�ӷֲ��ڲ�ͬ��̼ԭ���ϣ������������һ��̼ԭ����֧���ϣ�����˵����ȷ���ǣ�������
| A����̼�⻯����Ϊ2-��-1��3-���ϩ |
| B����̼�⻯�������嵥��1��1�ӳ�ʱ��������3�ֲ�ͬ�IJ��� |
| C����̼�⻯�������嵥��1��1�ӳ�ʱ��������2�ֲ�ͬ�IJ��� |
| D����̼�⻯���������������ӳ�ʱ�������������� |
���㣺�й��л������ʽȷ���ļ���
ר�⣺�������������ȼ�չ���
����������ȡһ������ȫȼ�գ�ʹȼ�պ������ͨ������ܣ����������0.72gΪȼ������ˮ�����������ʵ���=
=0.04mol����ͨ��ʯ��ˮ��ʯ��ˮ����2.2gΪȼ�����ɶ�����̼�������������ʵ���=
=0.05mol�����̼�⻯���������C��Hԭ����Ŀ֮��=0.05mol��0.04mol��2=5��8�������ʽΪC5H8��
�ڸ�̼�⻯������壩���ܶ�����ͬ�����������ܶȵ�34����������Է�������=34��2=68�����л������ʽΪC5H8���䲻���Ͷ�=
=2��
�۸�̼�⻯����0.1mol�ܺ�32g����ӳɷ�Ӧ��������ʵ���=
=0.2mol��̼�⻯������������ʵ���֮��Ϊ1��2����̼�⻯��������к���2��C=C����1��-C��C-����
�ܾ��������ڢ۵��������У���ԭ�ӷֲ��ڲ�ͬ��̼ԭ���ϣ�˵��̼�⻯��������к���2��C=C���������������һ��̼ԭ����֧���ϣ��ʸ�̼�⻯����Ľṹ��ʽΪ��CH2=CH-C��CH3��=CH2��
| 0.72g |
| 18g/mol |
| 2.2g |
| 44g/mol |
�ڸ�̼�⻯������壩���ܶ�����ͬ�����������ܶȵ�34����������Է�������=34��2=68�����л������ʽΪC5H8���䲻���Ͷ�=
| 5��2+2-8 |
| 2 |
�۸�̼�⻯����0.1mol�ܺ�32g����ӳɷ�Ӧ��������ʵ���=
| 32g |
| 160g/mol |
�ܾ��������ڢ۵��������У���ԭ�ӷֲ��ڲ�ͬ��̼ԭ���ϣ�˵��̼�⻯��������к���2��C=C���������������һ��̼ԭ����֧���ϣ��ʸ�̼�⻯����Ľṹ��ʽΪ��CH2=CH-C��CH3��=CH2��
���
�⣺����ȡһ������ȫȼ�գ�ʹȼ�պ������ͨ������ܣ����������0.72gΪȼ������ˮ�����������ʵ���=
=0.04mol����ͨ��ʯ��ˮ��ʯ��ˮ����2.2gΪȼ�����ɶ�����̼�������������ʵ���=
=0.05mol�����̼�⻯���������C��Hԭ����Ŀ֮��=0.05mol��0.04mol��2=5��8�������ʽΪC5H8��
�ڸ�̼�⻯������壩���ܶ�����ͬ�����������ܶȵ�34����������Է�������=34��2=68�����л������ʽΪC5H8���䲻���Ͷ�=
=2��
�۸�̼�⻯����0.1mol�ܺ�32g����ӳɷ�Ӧ��������ʵ���=
=0.2mol��̼�⻯������������ʵ���֮��Ϊ1��2����̼�⻯��������к���2��C=C����1��-C��C-����
�ܾ��������ڢ۵��������У���ԭ�ӷֲ��ڲ�ͬ��̼ԭ���ϣ�˵��̼�⻯��������к���2��C=C���������������һ��̼ԭ����֧���ϣ��ʸ�̼�⻯����Ľṹ��ʽΪ��CH2=CH-C��CH3��=CH2��
A����̼�⻯����ΪCH2=CH-C��CH3��=CH2������Ϊ2-��-1��3-����ϩ����A����
B�����嵥��1��1�ӳ�ʱ���Է���1��2-�ӳɻ�1��4-�ӳɣ�����3�ֲ�ͬ�IJ��BrCH2CH��Br��-C��CH3��=CH2��CH2=CH-CBr��CH3��CH2Br��BrCH2CH=C��CH3��CH2Br����B��ȷ��
C����B�з�����֪�����嵥��1��1�ӳ�ʱ���Է���1��2-�ӳɻ�1��4-�ӳɣ�����3�ֲ�ͬ�IJ����C����
D����̼�⻯���������������ӳ�ʱ����CH3CH2-C��CH3��CH3��Ϊ�����飬��D����
��ѡB��
| 0.72g |
| 18g/mol |
| 2.2g |
| 44g/mol |
�ڸ�̼�⻯������壩���ܶ�����ͬ�����������ܶȵ�34����������Է�������=34��2=68�����л������ʽΪC5H8���䲻���Ͷ�=
| 5��2+2-8 |
| 2 |
�۸�̼�⻯����0.1mol�ܺ�32g����ӳɷ�Ӧ��������ʵ���=
| 32g |
| 160g/mol |
�ܾ��������ڢ۵��������У���ԭ�ӷֲ��ڲ�ͬ��̼ԭ���ϣ�˵��̼�⻯��������к���2��C=C���������������һ��̼ԭ����֧���ϣ��ʸ�̼�⻯����Ľṹ��ʽΪ��CH2=CH-C��CH3��=CH2��
A����̼�⻯����ΪCH2=CH-C��CH3��=CH2������Ϊ2-��-1��3-����ϩ����A����
B�����嵥��1��1�ӳ�ʱ���Է���1��2-�ӳɻ�1��4-�ӳɣ�����3�ֲ�ͬ�IJ��BrCH2CH��Br��-C��CH3��=CH2��CH2=CH-CBr��CH3��CH2Br��BrCH2CH=C��CH3��CH2Br����B��ȷ��
C����B�з�����֪�����嵥��1��1�ӳ�ʱ���Է���1��2-�ӳɻ�1��4-�ӳɣ�����3�ֲ�ͬ�IJ����C����
D����̼�⻯���������������ӳ�ʱ����CH3CH2-C��CH3��CH3��Ϊ�����飬��D����
��ѡB��
���������⿼���л����ƶϡ�ϩ�������ʵȣ��Ѷ��еȣ�ע�����չ����ϩ������ļӳɷ�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
�����ϩ���Ҵ��Ľṹ�����ʣ��Ʋ��ϩ����CH2=CH-CH2OH�����ܷ����Ļ�ѧ��Ӧ�ǣ�������
| A���ӳɷ�Ӧ |
| B��������Ӧ |
| C����Na��Ӧ |
| D����Na2CO3��Һ��Ӧ�ų�CO2 |
��100mL���ʵ���������Ϊ30%��ϡ�����м���100mL����ˮ���û����Һ�����ʵ�����������������
| A������15% | B������15% |
| C��С��15% | D����ȷ�� |
ij����X�ܴ��ε���Һ���û�������Y���ɴ��ƶϳ�����˵����ȷ���ǣ�������
| A��X��Y���ǽ���ʱ����Xһ����Y���� |
| B��Xһ�����ڽ����˳�����H��ǰ�� |
| C��X�ǽ���ʱ��Y�����ǽ���Ҳ�����Ƿǽ��� |
| D��X�Ƿǽ���ʱ��Y�����ǽ���Ҳ�����Ƿǽ��� |
��Ȼ����ʯ�͡�ú���ڵ����ϵ��̲��������ģ�������˵����ȷ���ǣ�������
�ٿ����õ��ˮ�ķ����Ƶ���������Դ
�ڿɿ�����ľ��Ұ������Դ
��Ӧ����̫���ܡ����ܵ�����Դ������Ӧ�÷��ܡ�ˮ�ܵȿ�������Դ��
�ٿ����õ��ˮ�ķ����Ƶ���������Դ
�ڿɿ�����ľ��Ұ������Դ
��Ӧ����̫���ܡ����ܵ�����Դ������Ӧ�÷��ܡ�ˮ�ܵȿ�������Դ��
| A���� | B���� | C���ڢ� | D���� |
�����л���������ȷ���ǣ�������
A��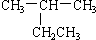 2-�һ����� 2-�һ����� |
B�� 1��2-�������� 1��2-�������� |
C�� ����ױ� ����ױ� |
D�� 2-��-2-��ϩ 2-��-2-��ϩ |
 ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���
ijЩ�Ͼ����Ͽɲ������з����������������ϸ���������ǿ�ȣ�ʹ�������õ������ʣ���ʵ��װ����ͼ�����Ⱦ۱�ϩ�����ϵõ��IJ������±���