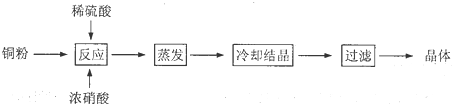
��Ŀ����
��2011?�Ϻ���ʵ������Ũ��Ϊ0.500mol/L�ı�����������Һ���ⶨδ֪Ũ�ȵ����ᣬ��������ʵ����̣�
��1���ζ���ʹ��ǰ����Ҫ���ζ��ܵĻ���
��2���ѱ�����������Һע���ñ�����������Һ��ϴ������ɫ�ֱ��ζ����У�ʹҺ��λ��
��3������ƿ�з���20.00mL�Ĵ�����Һ���ٵμ�2�η�̪��ҡ�ȣ��ñ�����������Һ�ζ����ߵα�ҡ����ƿ���۾�ע��
��4���ظ���2���ͣ�3������������¼���ݣ��ٴεζ���������������Һ���������±���
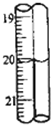 ��2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ���������ϱ��У����ݱ��е����ݼ���������Ũ��Ϊ
��2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ���������ϱ��У����ݱ��е����ݼ���������Ũ��Ϊ
��֪�����ȷŨ��Ϊ0.490mol/L����ʵ�����Ϊ
��5�������ʵ����ƫ�ߵIJ�����
a����ƿ������ˮϴ��������װ�������Һ
b����ʱ��Һ������ƿ��
c���ζ�ʱ��ɫ�ֱ��ζ����е�Һ�������ƿ�⣮
��1���ζ���ʹ��ǰ����Ҫ���ζ��ܵĻ���
�Ƿ�©Һ
�Ƿ�©Һ
��������ת�Ƿ�����2���ѱ�����������Һע���ñ�����������Һ��ϴ������ɫ�ֱ��ζ����У�ʹҺ��λ��
0�̶Ȼ�0�̶�����
0�̶Ȼ�0�̶�����
λ�ã���¼��������3������ƿ�з���20.00mL�Ĵ�����Һ���ٵμ�2�η�̪��ҡ�ȣ��ñ�����������Һ�ζ����ߵα�ҡ����ƿ���۾�ע��
��ƿ����Һ��ɫ
��ƿ����Һ��ɫ
�ı仯��ֱ���������һ������������Һ��ָʾ������ɫ����
��
ɫ��Ϊ�ۺ�
�ۺ�
ɫ�����ڰ��������Һ��ɫ�������仯��ֹͣ�ζ�����¼��������4���ظ���2���ͣ�3������������¼���ݣ��ٴεζ���������������Һ���������±���
| ���� | �ζ�ǰ��mL�� | �ζ���mL�� |
| 1 | 0.40 | 21.10 |
| 2 | 0.10 |
 ��2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ���������ϱ��У����ݱ��е����ݼ���������Ũ��Ϊ
��2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ���������ϱ��У����ݱ��е����ݼ���������Ũ��Ϊ0.496
0.496
mol/L����֪�����ȷŨ��Ϊ0.490mol/L����ʵ�����Ϊ
1.2
1.2
%����5�������ʵ����ƫ�ߵIJ�����
c
c
����д��ţ���a����ƿ������ˮϴ��������װ�������Һ
b����ʱ��Һ������ƿ��
c���ζ�ʱ��ɫ�ֱ��ζ����е�Һ�������ƿ�⣮
��������1���ζ���ʹ��ǰ����Ҫ�������Ƿ�©ˮ��
��2��װҺ��Ҫ��0��ʹҺ�洦��0�̶Ȼ�0�̶�����ijһ�̶ȼ�¼������
��3������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4���ζ��ܵĿ̶����϶��¿̶�������Ϊ0.01mL���ݴ˽��ͼ����������1��2��ƽ������V��NaOH�������������NaOH��Ӧ���C�����ᣩ������ʵ�����=
��100%��
��5������c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��2��װҺ��Ҫ��0��ʹҺ�洦��0�̶Ȼ�0�̶�����ijһ�̶ȼ�¼������
��3������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4���ζ��ܵĿ̶����϶��¿̶�������Ϊ0.01mL���ݴ˽��ͼ����������1��2��ƽ������V��NaOH�������������NaOH��Ӧ���C�����ᣩ������ʵ�����=
| ��c |
| c(��) |
��5������c�����⣩=
| c(��)��V(��) |
| V(����) |
����⣺��1���ζ���ʹ��ǰ����Ҫ�������Ƿ�©ˮ��
�ʴ�Ϊ���Ƿ�©Һ��
��2��װҺ��Ҫ��0��ʹҺ�洦��0�̶Ȼ�0�̶�����ijһ�̶ȼ�¼������
�ʴ�Ϊ��0�̶Ȼ�0�̶����£�
��3������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ����ɫͻ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ����ɫ���ۺ죻
��4����2�εζ���ζ��ܵĶ���20.10mL�����εζ���������������Һ������ֱ�Ϊ��19.70mL��20.00mL��
���1��2��ƽ������V��NaOH��=19.85mL��
HCl+NaOH�TNaCl+H2O
20.00mL��C�� HCl�� 19.85mL��0.500mol/L
��C��HCl��=
=0.496mol/L��
��֪�����ȷŨ��Ϊ0.490mol/L����ʵ�����Ϊ
��100%=1.2%��
�ʴ�Ϊ��0.496mol/L��1.2%��
��5��a����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��V��������Ӱ�죬����
c�����⣩=
��֪��c�����⣩��Ӱ�죬��a����
b����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС������V������ƫС������c�����⣩=
��֪��c�����⣩ƫС����b����
c���ζ�ʱ��ɫ�ֱ��ζ����е�Һ�������ƿ�⣬����V������ƫ����c�����⣩=
��֪��c�����⣩ƫ��c��ȷ��
��ѡ��c��
�ʴ�Ϊ���Ƿ�©Һ��
��2��װҺ��Ҫ��0��ʹҺ�洦��0�̶Ȼ�0�̶�����ijһ�̶ȼ�¼������
�ʴ�Ϊ��0�̶Ȼ�0�̶����£�
��3������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ����ɫͻ��Ϊ�ۺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ƿ����Һ��ɫ����ɫ���ۺ죻
��4����2�εζ���ζ��ܵĶ���20.10mL�����εζ���������������Һ������ֱ�Ϊ��19.70mL��20.00mL��
���1��2��ƽ������V��NaOH��=19.85mL��
HCl+NaOH�TNaCl+H2O
20.00mL��C�� HCl�� 19.85mL��0.500mol/L
��C��HCl��=
| 19.85mL��0.500mol/L |
| 20.00mL |
��֪�����ȷŨ��Ϊ0.490mol/L����ʵ�����Ϊ
| 0.496mol/L-0.490mol/L |
| 0.490mol/L |
�ʴ�Ϊ��0.496mol/L��1.2%��
��5��a����ƿ������ˮϴ��������װ�������Һ������Һ�����ʵ������䣬��V��������Ӱ�죬����
c�����⣩=
| c(��)��V(��) |
| V(����) |
b����ʱ��Һ������ƿ�⣬����Һ�����ʵ���ƫС������V������ƫС������c�����⣩=
| c(��)��V(��) |
| V(����) |
c���ζ�ʱ��ɫ�ֱ��ζ����е�Һ�������ƿ�⣬����V������ƫ����c�����⣩=
| c(��)��V(��) |
| V(����) |
��ѡ��c��
������������Ҫ�������к͵ζ��������������Լ����㣬�ѶȲ��������к͵ζ���ԭ���ǽ���ؼ���

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ
 ��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ�
��2011?�Ϻ���ʵ������ȡ�����������װ������ͼ��ʾ�������������������գ� ��2011?�Ϻ�����ͼ��ʵ������ȡHCl����NaCl+H2SO4��Ũ��
��2011?�Ϻ�����ͼ��ʵ������ȡHCl����NaCl+H2SO4��Ũ��