��Ŀ����
����Ŀ��ʵ�����Դ������κ�����Ϊԭ���Ʊ�����K2FeO4�IJ����������£�
���Ʊ�NaClOǿ���Ա�����Һ��
�ٽ�20 mL NaOH��Һ��������b�У���ˮԡ��ȴ��ͨ��Cl2�����裬ֱ����Һ��Ϊ����ɫ����������ɫ��������Ϊֹ��װ������ͼ��ʾ����
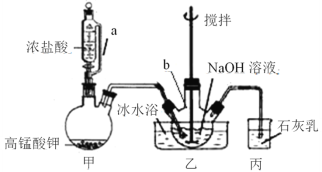
�ڽ����ñ���NaClO�����ձ���������ˮԡ�У��ּ��μ���20 g NaOH���岢���Ͻ��裬���ˣ���NaClOǿ���Ա�����Һ��
(1)��װ����a�ܵ�������_____________________________________________��
(2)д����װ���з�Ӧ�Ļ�ѧ����ʽ_____________________________________��
(3)ʯ�����������___________________________________________________��
(4)��Ӧ����������ˮԡ��ȴ��ԭ����___________________________________��
�ϳ�K2FeO4��
�ٳ�ȡ5.05 g Fe(NO3)3��9H2O����Է�������Ϊ404�����壬����ˮԡ�з����������������Һ�������Ͻ��裬��Ӧ1Сʱ����Һ�����Ϻ�ɫ����Na2FeO4����
�����ķ����ȥNa2FeO4ˮ��õ���Fe(OH)3���壬���ϲ���Һ�����Ϻ�ɫ����
����ڵ��ϲ���Һ�л�������KOH������Һ50.00mL����ˮԡ����5 min�����ˣ���K2FeO4����Է�������Ϊ198���ֲ�Ʒ��
�ܽ��ֲ�Ʒ�ؽᾧ�����������ϴ�ӣ����º�ɣ��ô���Ʒ2.13 g��
(5)�ϳ�Na2FeO4�����ӷ���ʽΪ____________________________________��
(6)���̢ۼ��뱥��KOH��Һ���ɵõ�K2FeO4�ֲ�Ʒ��ԭ����__________��
(7)�������ϴ�ӵ�Ŀ����__________________________________________��
(8)K2FeO4�IJ���Ϊ___________��������0.1%����
���𰸡�ƽ��ѹǿ��ʹ©���ڵ�Һ����˳������ 2KMnO4��16HCl=2KCl��MnCl2��5Cl2����8H2O ��ȥδ��Ӧ����������ֹ������Ⱦ���� ����������������������Һ��Ӧ���ɴ������� 2Fe 3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O K2FeO4���ܽ��С�� Na2FeO4�����뱥�� KOH ��Һ�������� K+��Ũ�ȣ�ʹ Na2FeO4+2KOH![]() K2FeO4+2NaOH ƽ�������ƶ����������� �����������ˮϴ�Ӳ�Ʒ���Լ��ٸ�����ص��ܽ���� 86.1%
K2FeO4+2NaOH ƽ�������ƶ����������� �����������ˮϴ�Ӳ�Ʒ���Լ��ٸ�����ص��ܽ���� 86.1%
��������
�������NaOH��Һ��ͨ�����ü�װ���Ƶõ����������裬ֱ����Һ��Ϊ����ɫ����������ɫ��������Ϊֹ��Ȼ����NaClO��Һ�м���NaOH���壬���ɵ�NaClOǿ���Ա�����Һ��
����NaClOǿ���Ա�����Һ�зִμ���Fe(NO3)3��9H2O(��Է�������Ϊ404)���壬������ˮԡ���ºͲ��Ͻ��裬���ɵ����Ϻ�ɫNa2FeO4��Һ�������ķ����ȥ����Fe(OH)3���壬Ȼ�����ϲ��л�������KOH������Һ�������˲��������ϴ�ӣ����º�ɣ��ø�����ء�
(1) ��װ���и��������Ũ���ᷢ��������ԭ��Ӧ��������������a�ܿ�ƽ��ѹǿ��ʹ©���ڵ�Һ����˳�����£�
(2) ��װ�����ø�����ع����ĩ��Ũ���ᷢ����Ӧ������ȡ��������Ӧ�Ļ�ѧ����ʽΪ2KMnO4��16HCl=2KCl��MnCl2��5Cl2����8H2O��
(3) �����ж�����Ⱦ������ʯ�����ȥδ��Ӧ��������ֹ������Ⱦ������
(4) �¶Ȳ�ͬʱ��������NaOH��Ӧ�IJ���Ҳ��ͬ�������£����ɴ������ƣ��¶ȸ�ʱ����Ӧ��������أ�6NaOH+3Cl2![]() 5NaCl+NaClO3+3H2O�������˴������ƵIJ�������Ӧ����������ˮԡ��ȴ����������������������Һ��Ӧ���ɴ������ƣ�
5NaCl+NaClO3+3H2O�������˴������ƵIJ�������Ӧ����������ˮԡ��ȴ����������������������Һ��Ӧ���ɴ������ƣ�
(5) ���������������������Ϊ�������(FeO42-)ͬʱ����Cl-��H2O����Ӧ�����ӷ���ʽΪ2Fe3++3ClO-+6OH-=2FeO42-+3Cl-+5H2O��
(6) ���¶��¸�����ص��ܽ�ȱȸ������Ƶ��ܽ��С�����뱥��KOH��Һ��������K+��Ũ�ȣ���С������ص��ܽ⣬�ٽ�������ؾ���������
(7) ������ؿ�����ˮ�������������ˮϴ�Ӳ�Ʒ���ɼ��ٸ�����ص��ܽ���ģ�
(8) 5.05g Fe(NO3)39H2O�����ʵ���Ϊn(Fe(NO3)39H2O)=0.0125mol��������Ԫ���غ��֪n(K2FeO4)=0.0125mol����m(K2FeO4)=0.0125mol��198g/mol=2.475g�������Ϊ=![]() ��100%=86.1%��
��100%=86.1%��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����2L���ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g��![]() CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 600 | 800 | 830 | 1000 | 1200 |
K | 0.25 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �� ��
��2���÷�ӦΪ ��Ӧ��ѡ��������������������������Ӧ��ƽ�������ͨ��һ����CO2����ƽ�ⳣ��K��________��CO2��ת����________��(����������������С������������)
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬�������� ����ѡ�۷֣���
a��������ѹǿ���� b�����������c(CO)���� c��v��(H2)��v��(H2O) d��c(CO2)��c(CO)
��4���� 600��ʱ���������г���1mol CO��1mol H2O����Ӧ�ﵽƽ���CO��ת������ ��