��Ŀ����
9���������������Ԫ�����ʵ������жϣ�����˵����ȷ���ǣ�������| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/10-10 m | 0.66 | 1.36 | 1.23 | 1.10 | 0.99 | 1.54 | 0.70 | 1.18 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
| A�� | ����������ˮ�������ԣ��ܣ��� | B�� | ��ˮ��Ӧ�ľ��ҳ̶ȣ��ڣ��� | ||
| C�� | ��̬�⻯����ȶ��ԣ��ܣ��� | D�� | �٢ޢ��γɵĻ����������ӻ����� |
���� ����Ԫ������ϼ�������������ȣ���ͻ��ϼ�=������-8��ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ���������������ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ�������������С�����Ӳ���Խ����ԭ�Ӱ뾶Խ���ݱ���Ԫ�ػ��ϼ�֪���ۢ�Ϊ��IA��Ԫ�ء���Ϊ��VIA��Ԫ�ء��ܢ����ڵ�VA��Ԫ�ء���Ϊ��IIA��Ԫ�ء���Ϊ��IIIA��Ԫ�أ�����ԭ�Ӱ뾶֪���٢ڢۢܢݢޢߢ�ֱ���O��Mg��Li��P��Cl��Na��N��BԪ�أ�
A��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��
B��Ԫ�صĽ�����Խǿ���䵥����ˮ��ӦԽ���ң�
C��Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ��
D���٢ޢ��γɵĻ�����ΪNaNO3��NaNO2���������Ӽ���
��� �⣺����Ԫ������ϼ�������������ȣ���ͻ��ϼ�=������-8��ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ���������������ͬһ����Ԫ��ԭ�Ӱ뾶����ԭ�������������С�����Ӳ���Խ����ԭ�Ӱ뾶Խ���ݱ���Ԫ�ػ��ϼ�֪���ۢ�Ϊ��IA��Ԫ�ء���Ϊ��VIA��Ԫ�ء��ܢ����ڵ�VA��Ԫ�ء���Ϊ��IIA��Ԫ�ء���Ϊ��IIIA��Ԫ�أ�����ԭ�Ӱ뾶֪���٢ڢۢܢݢޢߢ�ֱ���O��Mg��Li��P��Cl��Na��N��BԪ�أ�
A��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ���ܡ��ݷֱ���P��ClԪ�أ��ǽ�����Cl��P����������������ˮ�������ԣ��ܣ��ݣ���A����
B��Ԫ�صĽ�����Խǿ���䵥����ˮ��ӦԽ���ң������ԣ��ڣ��ޣ������䵥����ˮ��Ӧ���ҳ̶ȣ��ڣ��ޣ���B����
C��Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ���ǽ����ԣ��ܣ��ߣ����⻯����ȶ��ԣ��ܣ��ߣ���C����
D���٢ޢ��γɵĻ�����ΪNaNO3��NaNO2�������Ӻ��������֮��������Ӽ���N-Oԭ��֮����ڹ��ۼ����������γ����ӻ������D��ȷ��
��ѡD��
���� ���⿼��λ�ýṹ�������ϵ��Ӧ�ã����ؿ���ѧ�������ж���������ȷԪ�ػ��ϼ�����������ԭ�Ӱ뾶��С���ɵȼ��ɽ��֪��ͬһ���ڡ�ͬһ����Ԫ�����ʵݱ���ɣ���Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ԭ�Ӱ뾶��A��B��D��C | B�� | ԭ��������d��c��b��a | ||
| C�� | ���Ӱ뾶��C��D��B��A | D�� | ���ʵĻ�ԭ�ԡ�A��B��D��C |
| A�� | C3H8 | B�� | C2H6O | C�� | C | D�� | NaCl |
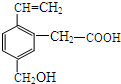 ij�л���Ľṹ��ͼ��ʾ�������л�����ܾ��е������ǣ�������
ij�л���Ľṹ��ͼ��ʾ�������л�����ܾ��е������ǣ����������ܷ���������Ӧ������ʹ��ˮ��ɫ�����ܸ�NaOH��Һ��Ӧ�����ܷ���������Ӧ��
���ܷ����ӳɷ�Ӧ�����ܷ���������Ӧ��
| A�� | ֻ�Тڢ� | B�� | ֻ�Тڢۢ� | C�� | ֻ�Т٢ڢۢܢ� | D�� | ȫ�� |
| A�� | C5H12��һ��ͬ���칹��ֻ������һ��һ�ȴ��� | |
| B�� | ���顢��ϩ�ͱ��ڹ�ҵ�϶���ͨ��ʯ�ͷ���õ� | |
| C�� | CH3-CH=CH-C��C-CF3���ӽṹ��6��̼ԭ�Ӳ����ܶ���һ��ֱ���� | |
| D�� | 75%��������������Ҵ���Һ������ҽ������ |
| A�� | SO2��SO3��H2SO4��MgSO4 | B�� | Fe��FeCl2��Fe��OH��2��Fe��OH��3 | ||
| C�� | Si��SiO2��H2SiO3��Na2SiO3 | D�� | Na��NaCl��NaOH��Na2CO3 |
| A�� | 1/22.4mol/L | B�� | V/33.6mol/L | C�� | V/22.4mol/L | D�� | 1/33.6mol/L |

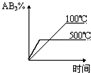



 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$
 ���ܵķ�Ӧ����ȡ����Ӧ����ˮ�ⷴӦ����
���ܵķ�Ӧ����ȡ����Ӧ����ˮ�ⷴӦ���� ��
�� ��
�� ��
��