��Ŀ����
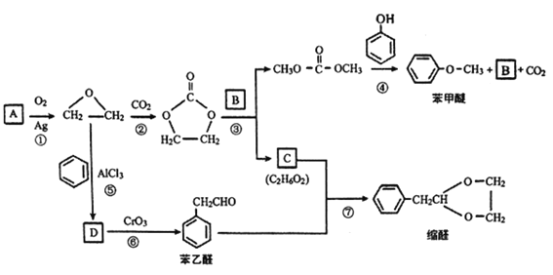
����Ŀ��A��C2H4���ǻ������л�����ԭ�ϡ���A�ͳ������л���ɺϳ�һ���������Ϻ�һ����ȩ�����ϣ�����ϳ�·����ͼ��ʾ�����ַ�Ӧ������ȥ����
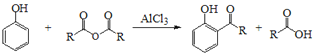
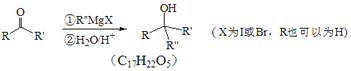
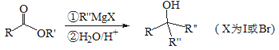
��֪��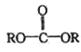 +
+![]() ��2ROH+
��2ROH+
�ش��������⣺
��1�� B�ķ���ʽ��__________ ����DΪ��ȡ�������廯��������������Ʒ�Ӧ��ÿ��D������ֻ����1����ԭ�ӣ� D����Ԫ�ص���������ԼΪ13.1%����D�Ľṹ��ʽΪ__________��
��2�� C�к��еĹ�����������_______________�����ķ�Ӧ������________________��
��3���ݱ�������Ӧ�����������£���NaHSO4��H2OΪ�������У���д���˷�Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
��4����д���������������ı���ȩ������ͬ���칹��Ľṹ��ʽ��______________________��
i .���б�����![]() �ṹ ii.�˴Ź���������4��壬�ҷ����֮��Ϊ3 : 2 : 2 : 1
�ṹ ii.�˴Ź���������4��壬�ҷ����֮��Ϊ3 : 2 : 2 : 1
��5����������EΪ�����ѵ�ͬϵ�����Է��������ȱ����Ѵ�14������ʹFeCl3��Һ��ɫ��E������ͬ���칹�干�У������������칹��__________�֡�
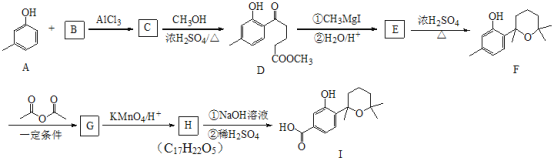
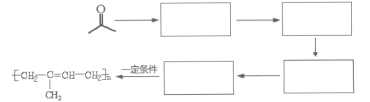
��6������ �ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ���ܼ������Լ��Ʊ�
�ĺϳ�·�ߣ�д����2-�ȱ���ͱ�Ҫ���ܼ������Լ��Ʊ� �ĺϳ�����ͼ��_____________________________��
�ĺϳ�����ͼ��_____________________________��
�ϳ�����ͼʾ�����£�![]()
���𰸡�CH4O  �ǻ� ������Ӧ
�ǻ� ������Ӧ 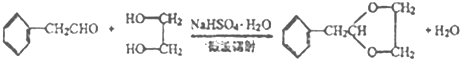
![]() ��
��![]() 9
9 
��������
A�ķ���ʽΪC2H4��AΪ��ϩ�����ṹ��ʽΪCH2=CH2���������⣨1����D��������Ʒ�Ӧ����D������ֻ��һ����ԭ�ӣ���D�к��У�OH��D���ڷ����廯�����D�к��б�����D����Է�������Ϊ1��16/13.1%=122�����ݱ���ȩ�Ƴ�D�Ľṹ��ʽΪ ��������ȩ�Ľṹ��ʽ���Ƴ�C�Ľṹ��ʽΪHOCH2CH2OH��������Ϣ���Ƴ�B�Ľṹ��ʽΪCH3OH���ݴ˷�����
��������ȩ�Ľṹ��ʽ���Ƴ�C�Ľṹ��ʽΪHOCH2CH2OH��������Ϣ���Ƴ�B�Ľṹ��ʽΪCH3OH���ݴ˷�����
A�ķ���ʽΪC2H4��AΪ��ϩ�����ṹ��ʽΪCH2=CH2���������⣨1����D��������Ʒ�Ӧ����D������ֻ��һ����ԭ�ӣ���D�к��У�OH��D���ڷ����廯�����D�к��б�����D����Է�������Ϊ1��16/13.1%=122�����ݱ���ȩ�Ľṹ��ʽ�Ƴ�D�Ľṹ��ʽΪ ��������ȩ�Ľṹ��ʽ���Ƴ�C�Ľṹ��ʽΪHOCH2CH2OH��������Ϣ���Ƴ�B�Ľṹ��ʽΪCH3OH��
��������ȩ�Ľṹ��ʽ���Ƴ�C�Ľṹ��ʽΪHOCH2CH2OH��������Ϣ���Ƴ�B�Ľṹ��ʽΪCH3OH��
��1����������������B�Ľṹ��ʽΪCH3OH��������ʽΪCH4O��D��������Ʒ�Ӧ����D������ֻ��һ����ԭ�ӣ���D�к��У�OH��D���ڷ����廯�����D�к��б�����D����Է�������Ϊ1��16/13.1%=122�����ݱ���ȩ�Ľṹ��ʽ���Ƴ�D�Ľṹ��ʽΪ  ��
��
��2��C�Ľṹ��ʽΪHOCH2CH2OH�����еĹ��������ǻ������ݺϳ�·�ߣ���Ӧ���DZ��Ҵ��������ɱ���ȩ����Ӧ����Ϊ������Ӧ��
��3����Ӧ���DZ���ȩ��HOCH2CH2OH��Ӧ������ȩ������ѧ��Ӧ����ʽΪ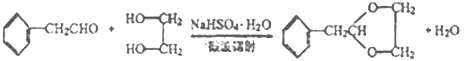 ��
��
��4��������Ϣ���˴Ź���������4��壬˵���ǶԳƽṹ�������֮��Ϊ3��2��2��1��˵����ͬ��ѧ��������ԭ�ӵĸ�����Ϊ3��2��2��1�����������Ľṹ��ʽΪ ![]() ��
��![]() ��
��
��5����������EΪ�����ѵ�ͬϵ�����Է��������ȱ����Ѵ�14���������ʽΪC8H10O��E��ͬ���칹����ʹFeCl3��Һ��ɫ��˵�����з��ǻ������豽�����У�OH����C2H5����ȡ���������ڼ�����ֽṹ�����豽�����У�OH����CH3����CH3����ȡ����������������λ���ǻ���2�ֽṹ���������ڼ�λ���ǻ���3�ֽṹ���������ڶ�λ���ǻ���1�ֽṹ��һ����9�ֽṹ��
��6�����ݷ�Ӧ�ڣ��Ƴ�����Ŀ����ԭ����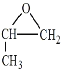 ������·�ߢ٣�����ϩ��O2��Ag�����������������ɻ������飬�������
������·�ߢ٣�����ϩ��O2��Ag�����������������ɻ������飬������� ��ԭ����CH3CH=CH2�����ɱ�ϩ����2���ȱ��鷢����ȥ��Ӧ���ϳ�·��Ϊ
��ԭ����CH3CH=CH2�����ɱ�ϩ����2���ȱ��鷢����ȥ��Ӧ���ϳ�·��Ϊ![]() ��
��

����Ŀ��ij�¶��£��� 2 L �����ܱ������� 3 �����ʼ���з�Ӧ��X��Y��Z �����ʵ�����ʱ��ı仯������ͼ��ʾ����Ӧ�� t1 min ʱ�ﵽƽ�⡣
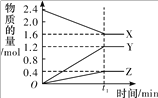
��1����д���÷�Ӧ�Ļ�ѧ����ʽ��_____��
��2����������Ӧ��X��Y��Z �ֱ�Ϊ NH3��H2��N2���ڴ� t1 min ʱ���ڣ���H2 ��ʾ�÷�Ӧ��ƽ������ v��H2��Ϊ_____��
��3���� 1mol ���ۼ��������յ��������±���
���ۼ� | H��H | N��N | N��H |
���յ�����/kJ | 436 | 946 | 391 |
1mol N2 ��ȫ��ӦΪNH3_____������ջ�ų���_____kJ ��������ʵ�ϣ��� 1molN2 ��3molH2 ���ڷ�Ӧ�����У�ʹ���dz�ַ�Ӧ����Ӧ�������仯��С�ڼ���ֵ��ԭ����_________��
��4�������������жϸ÷�Ӧ�ﵽƽ��״̬����_____������ĸ���ţ���
A �����ڸ�������ֵ������������ٷ����ı�
B ����Ӧ�������淴Ӧ�������
C ������������ܶȲ��ٷ����ı�
D ��������ƽ����Է����������ٷ����ı�
����Ŀ��X��Y��Z��W��R��P��Q�Ƕ���������Ԫ�أ�������Ϣ�����ʾ��
X | Y | Z | W | R | P | Q | |
ԭ�Ӱ뾶/nm | 0.154 | 0.074 | 0.099 | 0.075 | 0.143 | ||
��Ҫ���ϼ� | -4��+4 | -2 | -1��+7 | -3��+5 | +3 | ||
���� | �����Ӻ������� | ���ǽ������ϵ����� | ��ɫ��Ӧ�ʻ�ɫ |
������������⣺
��1��W�����ڱ��е�λ����___��X��P�γɵĻ�����ĵ���ʽ___��
��2��R����Ȼ������������Ϊ35��37�����ֺ��أ�����֮��Ĺ�ϵ�ǻ�Ϊ___��
��3��Z��Q��Ԫ�ص����������ˮ����֮�䷢����Ӧ�����ӷ���ʽΪ___��
��4��Y��R��ȣ��ǽ����Խ�ǿ����___����Ԫ�ط��ű�ʾ�����������з�����֤����һ���۵���___������ĸ��ţ���
A.������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬
B.R��Y���⻯���ȶ��Բ�ͬ
.Y��R�γɵĻ������еĻ��ϼ�
D.R��Y���������ˮ���������ǿ��
��5���õ���ʽ��ʾ��Z��R��ɵĻ�������γɹ���___��
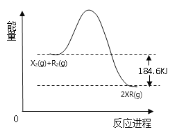
��6����ͼΪX2��R2ȼ�շ�Ӧ�������仯ʾ��ͼ������ݴ�ͼд���÷�Ӧ���Ȼ�ѧ����ʽΪ___�������ʻ�ѧʽ��ʾ����