��Ŀ����
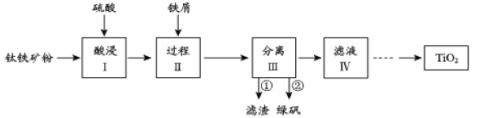
����Ŀ��������������Խ������Ϊ��������֮��ġ�������������������(��Ҫ�ɷ�ΪFeTiO3��������Fe2O3��SiO2������)�������Ʊ�TiO2��ͬʱ�õ�����Ʒ�̷�(FeSO4��7H2O)������������ͼ��ʾ��
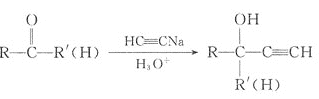
��֪����FeTiO3��2H2SO4��FeSO4��TiOSO4��2H2O
��TiO2����ˮ�⣬ֻ�ܴ�����ǿ������Һ��
��1�����I��Fe2O3��ϡ���ᷴӦ�����ӷ���ʽ��_____________________��
��2������II�м���������м��Ŀ����_____________________________��
��3������III�в���ڵõ��̷��IJ�����__________________________��
��4������ҺIV��ȡTiO2�Ĺ������£�
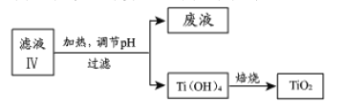
�����û�ѧƽ���ƶ�ԭ��������Һ������е�Ŀ�ģ�_______________��
����2Mg��TiCl4��Ti��2MgCl2��Ӧ��õ�Mg��MgCl2��Ti�Ļ����ɲ����������ķ�������õ�Ti�����������Ϣ������ȵ��¶��Ը���______�漴�ɡ�
TiCl4 | Mg | MgCl2 | Ti | |
�۵�/�� | -26.0 | 648.8 | 714 | 1667 |
�е�/�� | 136.4 | 1090 | 1412 | 3287 |
���𰸡�Fe2O3+6H+= 2Fe3++3H2O ��Fe3+��ԭΪFe2+ ����Ũ������ȴ�ᾧ������ �÷�ӦΪ���ȷ�Ӧ���¶�����ƽ�������ƶ�������������Ti(OH)4 1412
��������
��1��Fe2O3��ϡ���ᷴӦ������������ˮ��
��2����м���Խ������ӻ�ԭΪ�������ӣ�
��3�������̷����ܽ�����¶����߶�������ص������
��4���ٸ�������ӦΪ���ȷ�Ӧ�����з������ڸ���Mg��MgCl2��Ti���۷е������
��1��Fe2O3��ϡ���ᷴӦ������������ˮ�����ӷ���ʽΪFe2O3+6H+= 2Fe3++3H2O��
�𰸣�Fe2O3+6H+= 2Fe3++3H2O��
��2������II�м���������м��Ŀ���ǽ�Fe3+��ԭΪFe2+��
�𰸣���Fe3+��ԭΪFe2+��
��3���̷����ܽ�����¶����߶�������III�в���ڵõ��̷��IJ����Ǽ���Ũ������ȴ�ᾧ�����ˣ�
�𰸣�����Ũ������ȴ�ᾧ�����ˣ�
��4���ٸ÷�ӦΪ���ȷ�Ӧ���¶�����ƽ�������ƶ�������������Ti(OH)4 ��
�𰸣��÷�ӦΪ���ȷ�Ӧ���¶�����ƽ�������ƶ�������������Ti(OH)4��
��Mg��MgCl2�ķе������1412�棬��Ti���۵�Ϊ1667�棬���Ե��¶��Ը���1412��ʱMg��MgCl2���������ʽ��ȥ���õ�Ti��
�𰸣�1412��

����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ����KIO3�еĵ�Ԫ�� | ȡ����KIO3������������������ˮ���ټ��������ữ��AgNO3��Һ |
B | ����ʯ���ѽ����е���ϩ | ���������ͨ����������KMnO4��Һ�� |
C | �Ƚ�HClO��CH3COOH������ǿ�� | �����£���pH�Ʋⶨ���͵�NaClO��Һ�ͱ��͵� |
D | ���ὺ����Ʊ� | �� |
A.AB.BC.CD.D