��Ŀ����
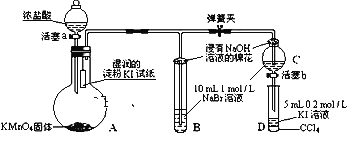
����Ŀ��2019��ŵ������ѧ�������ڿ�������ӵ�ط�������Խ������λ��ѧ�ҡ�����ӵ�صĹ㷺Ӧ��Ҫ������ط����Խ�Լ��Դ����������������﮵������������Ҫ����LiCoO2���������Ȳ�ڡ����������������ֿǵȣ������÷Ͼɵ�ص�һ�ֹ�������ͼ��ʾ��
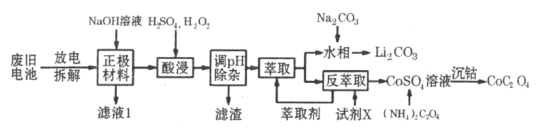
�ش��������⣺
(1)Liԭ�ӽṹʾ��ͼΪ_______��LiCoO2��Co�Ļ��ϼ���__________��
(2)��NaOH��Һ�����������ϵ����ӷ���ʽΪ____________________________��
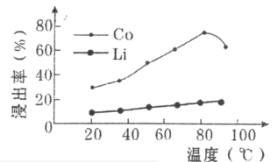
(3)�������������LiCoO2������Ӧ�����ӷ���ʽΪ___________________________�������������ز��������£��������ʱCo��LiԪ�صĽ��������¶ȵı仯����ͼ��ʾ�����¶ȸ���80��ʱCoԪ�ؽ������½���ԭ���У�
��Co2��ˮ��Ӿ磻��________________________________��
(4)����pH��Ŀ����ʹNi2����_______________(�����ӷ���)ȫ��������
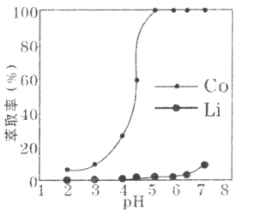
(5)����ȡ�����ڣ��ܡ����ȡ����ƽ��ʱ��ҺpH�Ĺ�ϵ����ͼ��ʾ��Ϊ��ʵ���ܡ�﮷���Ч���Ϻã�pHһ��ѡ��______________(������)���ҡ�
(6)����ȡ���͡�����ȡ���ɼ�ʾΪ![]() ������ȡ�����м�����Լ�X��___________________________(������)��
������ȡ�����м�����Լ�X��___________________________(������)��
(7)ȡCoC2O4����4.41g�ڿ����м�����300�棬�õ��ܵ�������2.41g��һ��������Ⱦ�����壬��÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
���𰸡� +3
+3 ![]()
![]() H2O2���ַֽ� Fe3�� 5 ������Һ
H2O2���ַֽ� Fe3�� 5 ������Һ ![]()
��������
�����̿�֪������NaOH��Һ���ݣ���Al����ܽ⣬���˺�õ�����Һ����NaAlO2������ΪLiCoO2�Լ�û�з�Ӧ����Ȳ�ڡ����������ֿǣ���H2SO4�����������ֿǻ��ܽ⣬�õ�Ni2����Fe2��������˫��ˮ�����Խ�Fe2��������Fe3�����ں���������У���֪Co�Ļ��ϼ۴�LiCoO2�еģ�3ת��Ϊ��2����H2O2����������ԭ��Ӧ�����백ˮ������ҺpH���ɳ�ȥNi2����Fe3��������ȡ�ķ���������Li����Co2�������յõ�Li2CO3��CoC2O4��
(1)Li��ԭ������Ϊ3����ԭ�ӽṹʾ��ͼΪ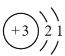 �����ݻ��ϼ۴�����Ϊ0��LiCoO2��Co�Ļ��ϼ�Ϊ��3��
�����ݻ��ϼ۴�����Ϊ0��LiCoO2��Co�Ļ��ϼ�Ϊ��3��
(2)���������к���Al��Al����NaOH��Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O=2AlO2����3H2����
(3)���������˫��ˮ����Li��Co��ת������Ӧ�����ӣ�LiCoO2��Co�Ļ��ϼ�Ϊ��3��������õ�CoC2O4��Co�Ļ��ϼ�Ϊ��2�����ϼ۽��ͣ���Ҫһ����ԭ����˫��ˮ��������ԭ���������ķ�ӦΪ2LiCoO2��H2O2��6H��=2Li2����2Co2����O2����4H2O���¶ȸ���80��ʱ��CoԪ�ؽ������½���H2O2��ֽ⣬ʹ��Co�������½���
(4)��H2SO4�����������ֿǻ��ܽ⣬�õ�Ni2����Fe2��������˫��ˮ�����Խ�Fe2��������Fe3��������pH�����Խ�Ni2����Fe3��ת��ΪNi(OH)2��Fe(OH)3�Ա��ȥ��
(5)����ȡ�����ڣ�Ϊ��ʵ���ܡ�﮷��롣��ͼ�п�֪��pH���ڵ���5ʱ��Co��ȡ���Ѿ��ﵽ100%����pHԽ��LiҲ�ᱻ��ȡ��ʹ��Li��Co������ȫ���룻���pHһ��ѡ��5��
(6)������ȡ��õ�CoSO4��Һ����֪�����ӦΪH2SO4��X������Ϊ������Һ��
(7)ȡCoC2O4����4.41g�ڿ����м�����300�棬�õ��ܵ�������2.41g����n(CoC2O4)= 4.41g��147g��mol��1=0.03mol������Co�غ㣬Co���������У�O������Ϊ2.41��0.03mol��59g��mol��1=0.64g����n(O)=0.64g��16g��mol��1=0.4mol����������Co��O�����ʵ���֮��Ϊ0.03mol��0.04mol=3:4�����������Ļ�ѧ��ΪCo3O4������ԭ���غ㣬��һ����������ΪCO2����ѧ����ʽΪ3CoC2O4��2O2![]() Co3O4��6CO2��
Co3O4��6CO2��

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�