��Ŀ����
��12�֣�����Ҫ����д��
��1���������ڿ�����ȼ�գ������ ɫ����ѧ����ʽ�� ��
��2�����������ƹ�������Թ��м�������ˮ���ټ��뼸�η�̪��Һ����Һ���ɫ���ܿ���ɫ��ԭ���ǣ� ���������ƹ�����ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
��
��3���մ��С�մ�ֱ��ͬŨ�ȵ����ᷴӦ����Ӧ�Ͽ��Ҿ��ҵ��� ���ѧʽ����С�մ������ᷴӦ�����ӷ���ʽΪ�� ��
��
��4�������ƺͽ����طֱ��ˮ��Ӧ�����У����з�Ӧ�Ͼ��ҵķ�Ӧ�Ļ�ѧ����ʽ�� ��
��1���������ڿ�����ȼ�գ������ ɫ����ѧ����ʽ�� ��
��2�����������ƹ�������Թ��м�������ˮ���ټ��뼸�η�̪��Һ����Һ���ɫ���ܿ���ɫ��ԭ���ǣ� ���������ƹ�����ˮ��Ӧ�Ļ�ѧ����ʽ��
 ��
����3���մ��С�մ�ֱ��ͬŨ�ȵ����ᷴӦ����Ӧ�Ͽ��Ҿ��ҵ��� ���ѧʽ����С�մ������ᷴӦ�����ӷ���ʽΪ��
 ��
����4�������ƺͽ����طֱ��ˮ��Ӧ�����У����з�Ӧ�Ͼ��ҵķ�Ӧ�Ļ�ѧ����ʽ�� ��
��12�֣�
��1���ƣ�1�֣���2Na+O 2====Na2O2��2�֣�
2====Na2O2��2�֣�
��2������������ǿ�����ԣ������������Ư���ԣ���2�֣���2Na2O2+2H2O=4NaOH+O 2����2�֣�
2����2�֣�
��3��NaHCO3��1�֣���HCO3��+ H+= H2O+CO2����2�֣�
H+= H2O+CO2����2�֣�
��4��2K+2H2O=2KOH+H2����2�֣�
��1���ƣ�1�֣���2Na+O
 2====Na2O2��2�֣�
2====Na2O2��2�֣���2������������ǿ�����ԣ������������Ư���ԣ���2�֣���2Na2O2+2H2O=4NaOH+O
 2����2�֣�
2����2�֣���3��NaHCO3��1�֣���HCO3��+
 H+= H2O+CO2����2�֣�
H+= H2O+CO2����2�֣���4��2K+2H2O=2KOH+H2����2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
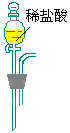
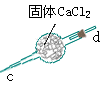

 CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼�������
CO3��Ʒ�е�NaHCO3�ֽ�ų��Ķ�����̼�������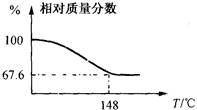
 ����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��
����Na2CO4�������ϴ����������Ŀ���Ƕ��������Ư�ס����������û�ѧ����ʽ��ʾ����ϴ��ԭ���� ��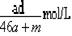
 8a L
8a L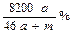
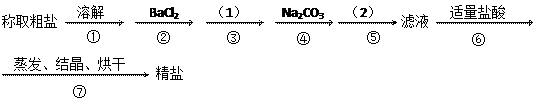
 �ںܿ͢ɷ�ߵ�_
�ںܿ͢ɷ�ߵ�_ �����ݣ�����ʵ��
�����ݣ�����ʵ�� �������Ӱ�죬��ԭ���ǣ�
�������Ӱ�죬��ԭ���ǣ�