��Ŀ����
7�����Ӹ���ʱ���õĺ�ҩΪ�Ȼ�泥����������������Ӵ������⣮�����ķ�Ӧ��ϵ�й����������ʣ�NH4C1��FeC13��N2��Fe2O3��Fe��X����1��дȫ�÷�Ӧ�Ļ�ѧ����ʽ������˫���ŷ�����÷�Ӧ�ĵ���ת�������6NH4C1+4Fe2O3�T3N2+6Fe+2FeC13+12H2O��
��2������������Ӧ��������NH4Cl����Ӧ��4mol���������ܵõ� ���ʧȥ���õ�����24mol����
��3����Ӧ�б�����������11.2L����״����������ʱ������ԭ�����ʵ����ʵ���Ϊ0.5mol��
��4�����Ȱ���NH2Cl����������������Һ�ȴ�������ˮ��ͬʱͨ������������������Ӧ��Cl2+NH3�TNH2Cl+HCl��NH2Cl����ˮ��Ӧ���ɿ���ɱ�����������ʣ��÷�Ӧ��Ԫ�صĻ��ϼ۲��䣮
��NH2Cl��ˮ��Ӧ�Ļ�ѧ����ʽ��NH2Cl+H2O=NH3+HClO��NH2Cl+2H2O=NH3•H2O+HClO��
����Cl2+NH3=NH2Cl+HCl�У�ÿ����11.2L Cl2����״���£���ת�Ƶ���0.5 mol��
���� ��1����ҩΪ�Ȼ�泥������������������Ӵ��������֪��Ӧ�����Ԫ���غ�������X��FeԪ�صĻ��ϼ۽��͡�NԪ�صĻ��ϼ����ߣ����õ����غ㼰�����غ㶨������ƽ��Ӧ��
��2����Ӧ�У�NԪ�ػ��ϼ����ߣ���������FeԪ�ػ��ϼ۽��ͣ���+3�۽��͵�0�ۣ�����ԭ��Fe2O3Ϊ�������������ɵ���ʱת�Ƶĵ��������㣻
��3����6NH4Cl+4Fe2O3�T6Fe+2FeCl3+3N2��+12H2O��֪�������������������õ��������ù�ϵΪ��3N2��3Fe2O3�����ݻ�ѧ����ʽ�������������õ������������㣻
��4����NH2Cl�ܲ���ˮ����������HClO�������ɰ������ݴ���д����ʽ��
ֻ��ClԪ�ػ��ϼ۷����仯�����������ʵ���Ϊ0.5mol�����ϼ۷ֱ���0�۱仯Ϊ-1�ۡ�+1�ۣ�
��� �⣺��1�����ݺ�ҩΪ�Ȼ�泥����������������Ӵ��������֪��Ӧ��ΪNH4Cl��Fe2O3����÷�Ӧ��������ΪFeCl3��N2��Fe��X������Ԫ���غ��֪X�к���H��OԪ�أ���XΪH2O���ɷ�Ӧ����������֪��NH4Cl+Fe2O3��Fe+FeCl3+N2��+H2O���÷�ӦFeԪ�صĻ��ϼ���+3�۽���Ϊ0��NԪ�صĻ��ϼ���-3�����ߵ�0��
���ݵ����غ��֪���õ�����=ʧȥ������=18e-����6NH4Cl+Fe2O3��6Fe+FeCl3+3N2��+H2O���������غ㶨�ɿ�֪����ƽ�Ļ�ѧ��ӦΪ6NH4Cl+4Fe2O3�T6Fe+2FeCl3+3N2��+12H2O������ת�����£�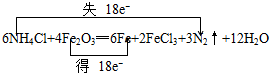
�ʴ�Ϊ��12H2O��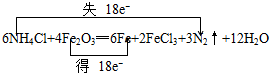 ��
��
��2����Ӧ6NH4Cl+4Fe2O3�T6Fe+2FeCl3+3N2��+12H2O�У�NԪ�ػ��ϼ����ߣ���������FeԪ�ػ��ϼ۽��ͣ���+3�۽��͵�0�ۣ�����ԭ��Fe2O3Ϊ���������ɷ���ʽ��֪����4molFe2O3�μӷ�Ӧʱ����6mol����ԭ����4molFe2O3������ԭ��Ӧ�õ�24mol���ӣ�
�ʴ�Ϊ��NH4Cl���õ���24��
��3��11.2L����״���������壬�����ʵ���Ϊ$\frac{11.2L}{22.4L/mol}$=0.5mol����6NH4Cl+4Fe2O3�T6Fe+2FeCl3+3N2��+12H2O��֪�������������������õ��������ù�ϵΪ��3N2��3Fe2O3����ԭ�����ʵ�����Ϊ0.5mol��160g/mol=80g���ʴ�Ϊ��0.5��
��4����NH2Cl�ܲ���ˮ������ǿ�����Ե����ʣ�ӦΪHClO����������ɱ�������ã�����ʽΪNH2Cl+H2O=NH3+HClO��NH2Cl+2H2O=NH3•H2O+HClO���ʴ�Ϊ��NH2Cl+H2O=NH3+HClO��NH2Cl+2H2O=NH3•H2O+HClO��
��ֻ��ClԪ�ػ��ϼ۷����仯�����������ʵ���Ϊ0.5mol�����ϼ۷ֱ���0�۱仯Ϊ-1�ۡ�+1�ۣ���ת�Ƶ���0.5mol���ʴ�Ϊ��0.5��
���� ���⿼��������ԭ��Ӧ����ƽ���йؼ��㣬���ݵ����غ㼰�����غ㶨�ɵó���ѧ��Ӧ����ʽ�ǽ����Ĺؼ�����ע�⣨2����ѧ�������ѵ���״��㣬4mol Fe2O3���뷴Ӧ�����������������Ѷ��еȣ�

| A�� | ̼������Һ | B�� | �Ȼ�����Һ | ||
| C�� | ���Ը��������Һ | D�� | ��ˮ |
| A�� | ���������ԭ��ѧ˵ | |
| B�� | �Ž��з������Ԫ�������� | |
| C�� | ��ķ�����ֵ��ӣ������ԭ�ӽṹ���ģ�� | |
| D�� | ���շ��������� |
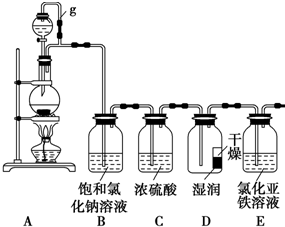 ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺
ij̽��С��Ϊ̽�����������ʣ����������ʵ��װ�ã���ش��������⣺ ijͬѧ������4mol•L-l�����ᣬ��ʵ����ֻ�����ֲ�ͬŨ�ȵ����
ijͬѧ������4mol•L-l�����ᣬ��ʵ����ֻ�����ֲ�ͬŨ�ȵ���� ����Ԫ���������е�λ���ǵڶ����ڵ�VIA�壮
����Ԫ���������е�λ���ǵڶ����ڵ�VIA�壮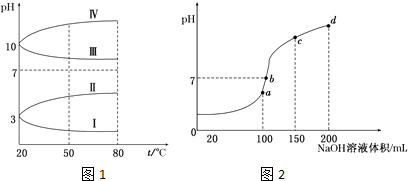
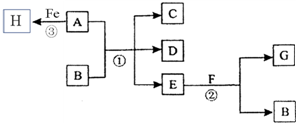
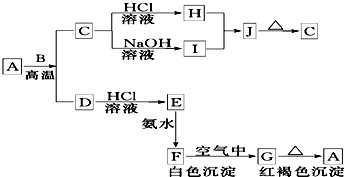 A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�CҲ��һ�ֽ���������������̬��C�õ�B���ʣ�J��һ��������ˮ�İ�ɫ���壬���Ⱥ��������ֽ⣮
A��һ�ֺ���ɫ���������B��D�ǽ������ʣ�CҲ��һ�ֽ���������������̬��C�õ�B���ʣ�J��һ��������ˮ�İ�ɫ���壬���Ⱥ��������ֽ⣮