��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺







�� ���� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ ��
 ��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� ��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳���� �� ��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ�� ��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ�� �� ��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1�U1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ� _��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1�U1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ� _�� a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4
a��MnO2 b��FeCl3 c��Na2SO3 d��KMnO4 ��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
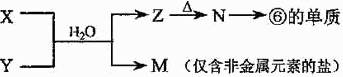
 X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ �� N���ĵ��ʵĻ�ѧ����ʽΪ ��
N���ĵ��ʵĻ�ѧ����ʽΪ �� �����£�Ϊʹ0.1 mol/L M ��Һ����M���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ�� ��
�����£�Ϊʹ0.1 mol/L M ��Һ����M���������������Ũ����ȣ�Ӧ����Һ�м���һ������Y��Һ�� ����1��Na��Al��O ��2��HNO3��H2CO3��H2SiO3 ��3��
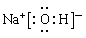 ��
��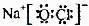 ��4��a b
��4��a b��5��Al3����3NH3��H2O=Al(OH)3����3NH4�� 2Al2O3(����)
 4Al��3O2�� ��Һ��pH����7
4Al��3O2�� ��Һ��pH����7���������ڱ�Ϊ��ģ�����ԭ�Ӱ뾶�Ƚϣ�����ǿ��������ʽ�����ӷ���ʽ�Լ�����ˮ���֪ʶ����1���ݢ�λ��ͬһ���ڣ��Ң����ڢ�ǰ�棬ԭ�Ӱ뾶��������һ���ڣ��Ȣݡ�����һ�����Ӳ㣬�ʰ뾶��С����2���ڢ�λ��ͬһ���壬����ķǽ�����ǿ����ۺ���������ǿ���ڢ�λ��ͬһ���ڣ��Ң��ںǽ�����ǿ����Ӧ������ǿ����3������Ԫ�طֱ�Ϊ�⡢�����ƺ��ȣ����Ӽ���Ȼ���������Σ����Թ��ۼ���Ӧ�����ַǽ�����ɡ���4��Һ̬H2O2������MnO2��FeCl3�ȴ��������·����ֽⷴӦ����5����ΪAl�����ƶ�ZΪAl(OH)3�����ȷֽ�ɲ���Al2O3���ٵ�⼴�ɵõ�������M�����ǽ������Σ���Ȼ��Σ�����X��YӦΪAlCl3��NH3��H2O�ķ�Ӧ������NH4Cl������NH4��ˮ�⣬��Ҫʹ��Ũ����Cl����ȣ���Ҫ����NH3��H2O���ɵ���غ�֪��c(NH4�� )+ c(H��)
 c(Cl��)+c(OH��),��c(NH4�� )
c(Cl��)+c(OH��),��c(NH4�� ) c(Cl��)����c(H��)= c(OH��)����pH=7��
c(Cl��)����c(H��)= c(OH��)����pH=7��
��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ
 ����
����
 ��Y��Z�����ڱ��е�λ����ͼ��ʾ��������˵����ȷ���� �� ��
��Y��Z�����ڱ��е�λ����ͼ��ʾ��������˵����ȷ���� �� ��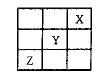
 ������ B
������ B �����������������
�����������������