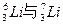��Ŀ����
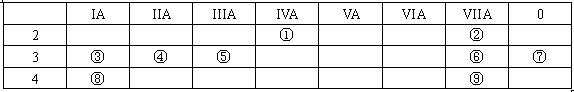
��17�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1���ڵ������̬�⻯����ӵĿռ乹��Ϊ__________________�ṹ��
��2���۵���̬�⻯���ȶ��ԱȢܵ���̬�⻯��______�����ǿ��������������ͬ����
 ��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ��
��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ��
 ��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________��
��5���ߵ�ԭ�ӽṹʾ��ͼΪ________________________��������������γɵľ�������Ϊ ���塣
��6���������������ݵ�����������Ӧˮ������Һ��Ӧ�����ӷ���ʽΪ ��
 ��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________��
��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________��
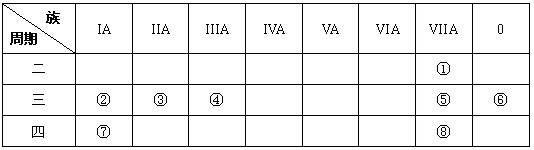
�� ���� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
��2���۵���̬�⻯���ȶ��ԱȢܵ���̬�⻯��______�����ǿ��������������ͬ����
 ��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ��
��3���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_________________�����ѧʽ�� ��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��____________________����5���ߵ�ԭ�ӽṹʾ��ͼΪ________________________��������������γɵľ�������Ϊ ���塣
��6���������������ݵ�����������Ӧˮ������Һ��Ӧ�����ӷ���ʽΪ ��
 ��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________��
��7���ɱ�������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���Һ̬�������ϡҺ�ױ�MnO2���ֽ⣬д����Ӧ�Ļ�ѧ����ʽ_________________����17�֣�
��1���������壨2�֣� ��2��ǿ��2�֣�
��3��HNO3>H2CO3>H2SiO3����H4SiO4�� ��2�֣�
��4��NaOH��Na2O2��NaClO ��2�֣�
��5��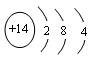 ��ԭ�� ����2�֣�
��ԭ�� ����2�֣�
��6��Al2O3+2OH��==2AlO2��+ H2O��3�֣�
��7��2H2O2 ="=" 2H2O + O2�� ��2�֣�
��1���������壨2�֣� ��2��ǿ��2�֣�
��3��HNO3>H2CO3>H2SiO3����H4SiO4�� ��2�֣�
��4��NaOH��Na2O2��NaClO ��2�֣�
��5��
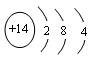 ��ԭ�� ����2�֣�
��ԭ�� ����2�֣���6��Al2O3+2OH��==2AlO2��+ H2O��3�֣�
|
��

��ϰ��ϵ�д�
 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ

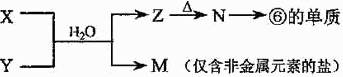
 ��1�����Ԫ�ط�����__________
��1�����Ԫ�ط�����__________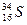 ���е��������� ���������� ������������� ������
���е��������� ���������� ������������� ������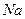 �γɵĻ�����ĵ���ʽ ���˻��������� ������ӻ�������ۻ��������
�γɵĻ�����ĵ���ʽ ���˻��������� ������ӻ�������ۻ��������