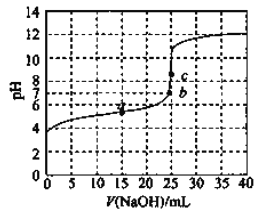
��Ŀ����
����Ŀ��ϴú��ѡú�������ų���ú��ʯ����Ҫ��Al2O3��SiO2��Fe2O3����ռ�ô�Ƭ���أ���ɻ�����Ⱦ��ij����������ú��ʯ�Ʊ��ۺ��Ȼ����������£�

��֪���ۺ��Ȼ�����[Al2(OH)nCl6-n]m��1��n��5��m��10������ҵ����PAC����һ�����͡���Ч�������;�ˮ����
��1�������Ŀ����___________________________��ʵ����Ҫ��500mL3.0 mol��L-1�����ᣬ����ʱ����Ҫ�IJ�����������Ͳ���ձ������������______________________��
��2����m=n=2��������PAC�Ļ�ѧ����ʽ��_____________________��
��3���Ӻ�PAC��ϡ��Һ�л��PAC�����ʵ�����������_______��_________��_________��
��4��Ϊ�˷�������2����Ԫ�صĺ�����ijͬѧ��ȡ5.000g����2���Ƚ���Ԥ����ʹ��Ԫ�ػ�ԭΪFe2+����������ƿ�����Ƴ�100mL��Һ��Ȼ����ȡ25.00mL������Һ����1.000��10-2mol��L-1KMnO4����Һ�ζ������ı���Һ20.00mL����֪��ӦʽΪFe2++MnO4��+H+��Fe3++Mn2++H2O��δ��ƽ�����жϵζ��յ��������_______________________������2����Ԫ�ص���������Ϊ________________��
���𰸡���ú��ʯ�е�Al2O3��Fe2O3�ܽ⣬��SiO2���� 500mL����ƿ����ͷ�ι� 4AlCl3+2Ca(OH)2![]() [Al2(OH)2Cl4]2+2CaCl2����4AlCl3+4H2O
[Al2(OH)2Cl4]2+2CaCl2����4AlCl3+4H2O![]() [Al2(OH)2Cl4]2+4HCl�� ����Ũ�� ��ȴ�ᾧ ���� �������һ�θ��������Һʱ����Һ����dz��ɫ���ڰ�����ڲ���ɫ 4.480%
[Al2(OH)2Cl4]2+4HCl�� ����Ũ�� ��ȴ�ᾧ ���� �������һ�θ��������Һʱ����Һ����dz��ɫ���ڰ�����ڲ���ɫ 4.480%
��������
��1�������Ŀ���dz�ȥSiO2����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ȷ����Ҫ��������
��2����Ӧ����AlCl3��Ca(OH)2����m=n=2�����������غ���ƽ���ɡ�
��3������Һ�л�ù���IJ�������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��4��KMnO4��Һ����ɫ������������ָʾ��������KMnO4��Һ����ɫ�仯�����жϵζ��յ㡣���ݵ����غ������Ԫ�صĺ�����
��1����Ϊú��ʯ����Ҫ��Al2O3��SiO2��Fe2O3�������������Ŀ�����ܽ����������������������������룻ʵ����Ҫ ��500mL3.0 mol��L-1�����ᣬ����ʱ����Ҫ�IJ�����������Ͳ���ձ������������500mL������ƿ��ʢ����Һ������ʱ�轺ͷ�ιܣ�
��2����������ͼ��֪�������������ƺ������ӳ�ȥ��ʣ����ҺΪ�Ȼ�����Һ���Ȼ������������Ƽ��ȷ�Ӧ����PAC���Ȼ��ƣ���ѧ����ʽ��4AlCl3+2Ca(OH)2![]() [Al2(OH)2Cl4]2+2CaCl2����4AlCl3+4H2O
[Al2(OH)2Cl4]2+2CaCl2����4AlCl3+4H2O![]() [Al2(OH)2Cl4]2+4HCl����
[Al2(OH)2Cl4]2+4HCl����
��3������Һ�еõ����ʵķ���������Ũ������ȴ�ᾧ�����ˣ����ԴӺ�PAC��ϡ��Һ�л��PAC�����ʵ�������������Ũ������ȴ�ᾧ�����ˣ�
��4����Ϊ�������ӵ���ҺΪdz����ɫ���������Ϊ��ɫ���ﵽ�ζ��յ�ʱ���μ�һ�θ��������Һ����ҺΪdz��ɫ���Ұ�����ڲ���ɫ�����ݵ�ʧ�����غ㣬���������ӵ�ϵ���Ǹ���������ӵ�5��������25.00mL��Һ��FeԪ�ص�������1.000��10-2mol��L-1��20��10-3L��5��56g/mol=0.056g����100mL��Һ��FeԪ�ص�������0.056g��4=0.224g�����Բ���2����Ԫ�ص���������Ϊ![]() ��100%=4.48%��
��100%=4.48%��

 �������ͬ����ϰϵ�д�
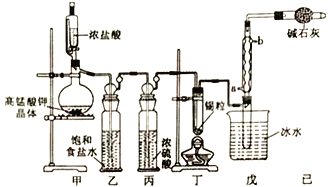
�������ͬ����ϰϵ�д�����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ����
�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ���������ܵĽ�ˮ��Ϊ___________���a����b������
��2���ü�װ����������MnO4-����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽___________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ��ٴٽ�����������Ӧ����_______________________________��
��4�����ȱ����װ�ã����ܲ����ĺ����___________________����װ�õ�������__________________��
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2 ����_______________�����ţ���
a.FeCl3��Һ������KSCN�� b.H2O2��Һ C.��ˮ d.AgNO3��Һ
��6����Ӧ����ȥ����1.19g����Ӧ������װ�õ��Թ����ռ���2.38 gSnCl4����SnCl4�IJ���Ϊ____________________��