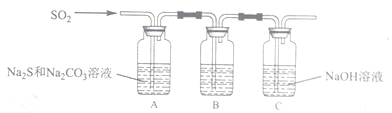
��Ŀ����
����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��������ж��ص����ʺ���;����ش��������⡣
��1��̼ԭ�Ӻ��������_____�ֲ�ͬ���˶�״̬��̼ԭ�ӵļ۵������γ�sp3�ӻ�����������ʽΪ_____��
��2��д��һ��CO32-�ĵȵ��������Ļ�ѧʽ_______����ռ乹��Ϊ_______��
��3���л���M(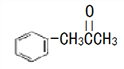 )��һ������������N(
)��һ������������N(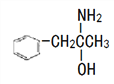 )��
)��
�ٷе㣺M_____N ����������������С��������
��M��̼ԭ���ӻ�����Ϊ_____����ͬ�ӻ����͵�̼ԭ����֮��Ϊ_____��
���л���N�г���ԭ��֮�������ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ_____��
��4����֪CaCO3���ȷֽ��¶�Ϊ900�棬SrCO3���ȷֽ��¶�Ϊ1172�����Դ�ԭ�ӽṹ�ĽǶȽ���CaCO3���ȷֽ��¶ȵ���SrCO3��ԭ��_____________��
��5��̼��һ��ͬ��������C60����������ϩ����һ�ָ߶ȶѳɵ���̼���ӡ������飨����ʽ��C8H8��![]() ���DZ�C60Լ��20��ϳɳ���һ�ֶԳ���������ӣ�������Ѻϳɳ�һ����������C60�ĸ����ͷ��Ӿ��壬�þ���ľ����ṹ����ͼ��ʾ����������������ԭC60����ķ��Ӽ��϶�С���ø����ͷ��Ӿ��������ö��ߵķ���ʽ�ɱ�ʾΪ______________��
���DZ�C60Լ��20��ϳɳ���һ�ֶԳ���������ӣ�������Ѻϳɳ�һ����������C60�ĸ����ͷ��Ӿ��壬�þ���ľ����ṹ����ͼ��ʾ����������������ԭC60����ķ��Ӽ��϶�С���ø����ͷ��Ӿ��������ö��ߵķ���ʽ�ɱ�ʾΪ______________��
��6��ʯī��̼��һ��ͬ�������壬����һ�־����ṹ�Ͳ��־�����������ͼ��
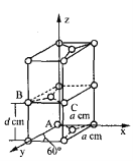
��ԭ����������������Ǿ�����ԭ�Ӽ�����λ�á�ʯī������̼ԭ��A��B����������ֱ�Ϊ��A(0��0��0)��B(0��1��1/2)����Cԭ�ӵ��������Ϊ_______________��
�ھ��������������������Ĵ�С����״����֪ʯī�����ױ߳�Ϊacm ������Ϊdcm�������ӵ�������ֵΪNA����ʯī���ܶ�Ϊ_____g��cm-3��д������ʽ���ɣ���
���𰸡� 6 ![]() SO3�� NO3- ��SiO32- ƽ�������� С�� sp2��sp3 7��2 N > O > C Ca2+�����Ӱ뾶С��Sr2+��Ca2+�����̼��������е������ӣ�ʹ̼������Ӹ��ֽ�Ϊ������̼ C8H8��C60 ��1��1��1/2��
SO3�� NO3- ��SiO32- ƽ�������� С�� sp2��sp3 7��2 N > O > C Ca2+�����Ӱ뾶С��Sr2+��Ca2+�����̼��������е������ӣ�ʹ̼������Ӹ��ֽ�Ϊ������̼ C8H8��C60 ��1��1��1/2�� ![]() g��cm-3
g��cm-3
��������(1).��Ϊû���˶�״̬��ͬ�ĵ��ӣ�����Cԭ�Ӻ�����6�в�ͬ�˶�״̬�ĵ��ӣ�̼ԭ�ӵ�2S���������2P��������ӻ���ͬʱ�����������ȵ���ռ��һ�������������������ʽΪ��![]() ��
��
(2). SO3��CO32-��Ϊ�ȵ����壬Sԭ����sp2�ӻ�����ɼ�, SO3����Ϊƽ���������η��ӣ�
(3). �ٶԽṹ���Ƶ��л���������Խ��е��Խ�ߣ����Էе㣺MС��N������ΪM�к���C-C�������Լ�C=O������M��C���ӻ���ʽΪsp2��sp3�ӻ��������ӻ���ʽ��̼ԭ�ӱ���Ϊ����6+1��:2=7:2 ������N��O��C�У���һ�����ܵ�˳��ΪN > O > C��
(4). ��SrCO3��CaCO3��Ϊ���Ӿ��壬SrCO3��CaCO3�������������ȣ�����Ca2+�뾶С��Sr2+�뾶������CaO�����ܴ���SrO�����ܣ�����Ca2+��Sr2+������̼��������е������ӽ�ϣ�ʹ̼������ӷֽ�ΪCO2��
(5).������C60��ĿΪ8*1/8+6*1/2=4����������������ԭC60����ķ��Ӽ��϶�У���������������ĿΪ4�������ʽΪC8H8��C60��
(6). ����B������ɵã�a=1��d=1/2������C������Ϊ��1��1��1/2��������ʯī�ľ����ṹ�þ�����Cԭ�ӵ�����Ϊ1+2��1/2+8��1/8+4��1/4=4,�������Ϊ2d��a��a��sin60��=![]() , ��ʯī���ܶ�Ϊ
, ��ʯī���ܶ�Ϊ![]() g��cm-3��
g��cm-3��