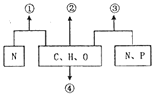
��Ŀ����
����Ŀ��������H��һ�����ϣ������ڽ����У���������·�ߺϳ���

�ش��������⣺
��1��11.2 L����״��������A�������г��ȼ�տ��Բ���88g CO2��45gH2O��A�ķ���ʽ��___________��
��2��D�����ƣ�ϵͳ������Ϊ____________________��
��3���ڴ���������1mol F��2 mol H2��Ӧ������3-����-1-������F�Ľṹ��ʽ��_______________��
��4����Ӧ���ķ�Ӧ������___________________��
��5����Ӧ���Ļ�ѧ����ʽΪ___________________��
��6����G������ͬ�Ĺ����ŵķ�����ͬ���칹����________�֣�����G��������HNMR��������壬�������Ϊ1��1��2��2��2���칹��Ľṹ��ʽ��___________________ ��
���𰸡�C4H10 2-����ϩ ![]() ��ȥ��Ӧ (CH3)2CHCH2OH+
��ȥ��Ӧ (CH3)2CHCH2OH+![]()
![]()
![]() +H2O 4
+H2O 4 ![]()
��������
���⡰ͻ�ƿڡ����ڶ�A��B��C�ĽṹA��������״����11.2LA�����ʵ���Ϊ0.5mol��ȼ������2mol CO2��2.5molH2O������ȼ�շ�Ӧ��̼����ԭ���غ��1molA�����к�4molC��10molH��A�ķ���ʽΪC4H10��Aͬ���칹����������(CH3CH2CH2CH3)���춡��[(CH3CH(CH3)2]���ٴ�һ�ȴ����������һ�ȴ�����ȥ��Ӧ����Ƕȷ�����Aֻ�����춡����NaOH����Һ��±������ȥ��Ӧ����������ȥ����D����![]() �����ݡ���֪��2������E��(CH3)2CHCH2OH������1molF��2molH2�ӳɺ���3-����-1-��������̼���Լ�F���롰����Cu(OH)2����Һ��Ӧ��ȷ��F��
�����ݡ���֪��2������E��(CH3)2CHCH2OH������1molF��2molH2�ӳɺ���3-����-1-��������̼���Լ�F���롰����Cu(OH)2����Һ��Ӧ��ȷ��F��![]() ������ȷ��G��
������ȷ��G��![]() ��Ũ����������E+G��H��Ӧ����������Ӧ���ɴ˷������
��Ũ����������E+G��H��Ӧ����������Ӧ���ɴ˷������
(1)11.2L����![]() =0.5mol������A�к�̼ԭ��
=0.5mol������A�к�̼ԭ��![]() ������ԭ��
������ԭ��![]() ����1molA�к�̼ԭ��2mol��0.5=4mol������ԭ��5mol��0.5=10mol������A�ķ���ʽΪC4H10��
����1molA�к�̼ԭ��2mol��0.5=4mol������ԭ��5mol��0.5=10mol������A�ķ���ʽΪC4H10��
(2)��ΪB��C����A��һ�ȴ�������NaOH����Һ�й��ȷ�����ȥ��Ӧ����ͬ������D��˵��B��C��̼����ͬ��A�ķ���ʽΪC4H10��A������ͬ���칹�壺�����飨CH3CH2CH2CH3�����춡��[CH3CH(CH3)2]�������춡���һ�ȴ�����2��CH2ClCH(CH3)2��CH3CCl(CH3)2������ȥ��Ӧ���ɵ��л����ﶼ��![]() ������D�Ľṹ��ʽΪ
������D�Ľṹ��ʽΪ![]() ��ϵͳ����Ϊ��2-����ϩ��
��ϵͳ����Ϊ��2-����ϩ��
(3)��F��������Cu(OH)2����Һ��Ӧ����F�����к���ȩ����-CHO��,1molF��2molH2�ӳɺ�IJ���Ϊ3-����-1-����������-OH��ȩ������õ�������1molH2ֻ����1mol>C=C<�ӳɵõ�������F�Ľṹ��ʽ![]() ��
��
(4) ��Ӧ����һ�ȴ��춡����NaOH����Һ���ȷ�����ȥ��Ӧ�����Է�Ӧ��������ȥ��Ӧ��
(5)�������������֪D��(CH3)2C=CH2��D��E���ϡ���֪(2)������EΪ(CH3)2CHCH2OH����(3)֪F�Ľṹ��ʽΪ![]() ��F��G�Ĺ�����2��������ȩ��������Ϊ�Ȼ������Σ�Ȼ���Ȼ������α��ữ�����Ȼ�������G�Ľṹ��ʽΪ
��F��G�Ĺ�����2��������ȩ��������Ϊ�Ȼ������Σ�Ȼ���Ȼ������α��ữ�����Ȼ�������G�Ľṹ��ʽΪ![]() ��(CH3)2CHCH2OH��
��(CH3)2CHCH2OH��![]() ��Ũ���������·���������Ӧ�����Է�Ӧ�ڵĻ�ѧ����ʽΪ��(CH3)2CHCH2OH+
��Ũ���������·���������Ӧ�����Է�Ӧ�ڵĻ�ѧ����ʽΪ��(CH3)2CHCH2OH+![]()
![]()
![]() +H2O��
+H2O��
(6)G�Ľṹ��ʽΪ![]() ������дͬ���칹������G������ͬ�Ĺ����ŵķ����ࡱ���ʣ�����дͬ���칹���к�һ��������һ��>C=C<��һ��-COOH���Ƚ�������>C=C<��ϣ����ԡ���Ч��ԭ�ӡ���˼����-COOH��-COOHȡ��λ����ͼ��
������дͬ���칹������G������ͬ�Ĺ����ŵķ����ࡱ���ʣ�����дͬ���칹���к�һ��������һ��>C=C<��һ��-COOH���Ƚ�������>C=C<��ϣ����ԡ���Ч��ԭ�ӡ���˼����-COOH��-COOHȡ��λ����ͼ��![]() �����Тپ���G���������G����4�֡�����HNMR��������壬�������Ϊ1��1��2��2��2���칹��Ľṹ��ʽΪ
�����Тپ���G���������G����4�֡�����HNMR��������壬�������Ϊ1��1��2��2��2���칹��Ľṹ��ʽΪ![]() ��
��

 ��������һ���þ�ϵ�д�
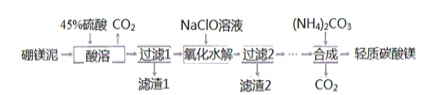
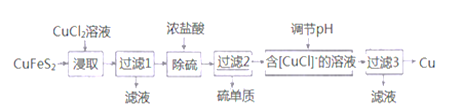
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�