��Ŀ����
����Ŀ��ij�¶��£���2L���ܱ������У�����1molX��g����2molY��g��������Ӧ��X��g��+m Y��g��![]() 3Z��g����ƽ��ʱ��X��Y��Z����������ֱ�Ϊ30%��60%��10%���ڴ�ƽ����ϵ�м���1molZ��g�����ٴδﵽƽ���X��Y��Z������������䡣������������ȷ����
3Z��g����ƽ��ʱ��X��Y��Z����������ֱ�Ϊ30%��60%��10%���ڴ�ƽ����ϵ�м���1molZ��g�����ٴδﵽƽ���X��Y��Z������������䡣������������ȷ����
A. m=2
B. ����ƽ���ƽ�ⳣ����ͬ
C. X��Y��ƽ��ת����֮��Ϊ1:1
D. �ڶ���ƽ��ʱ��Z��Ũ��Ϊ0.4 mol��L��1
���𰸡�D
��������
ij�¶��£���2L���ܱ������У�����1molX��g����2molY��g��������Ӧ��X��g��+m Y��g��![]() 3Z��g����ƽ��ʱ��X��Y��Z����������ֱ�Ϊ30%��60%��10%���ڴ�ƽ����ϵ�м���1molZ��g������ɵ�ЧΪ����Чƽ����ϵ�ϣ��ںϲ�˲��X��Y��Z������������䣬����λ�������ϵ�����������࣬������������ԭ��ƽ��Ӧ��ʹ��λ����ڷ���������С�����ƶ������ٴδﵽƽ���X��Y��Z������������䣬��˵��m+1=3����m=2��A����ȷ��ͬһ��ѧ��Ӧ��ƽ�ⳣ��ֻ���¶��йأ�����ƽ���¶Ȳ��䣬������ƽ���ƽ�ⳣ����ͬ��B����ȷ��m=2������ʼ��X��Y֮��Ϊ1:2����Ӧ�������ɷ���ʽ��֪��Ӧ��X��Y֮��Ϊ1:2����X��Y��ƽ��ת����֮��Ϊ1:1��C����ȷ��m=2����÷�ӦΪ��Ӧǰ��������������ķ�Ӧ���ʵڶ���ƽ��ʱZ�����ʵ���Ϊ��4��10%=0.4mol����Z��Ũ��Ϊ0.4mol��2L=0.2mol/L����D�������ѡD��
3Z��g����ƽ��ʱ��X��Y��Z����������ֱ�Ϊ30%��60%��10%���ڴ�ƽ����ϵ�м���1molZ��g������ɵ�ЧΪ����Чƽ����ϵ�ϣ��ںϲ�˲��X��Y��Z������������䣬����λ�������ϵ�����������࣬������������ԭ��ƽ��Ӧ��ʹ��λ����ڷ���������С�����ƶ������ٴδﵽƽ���X��Y��Z������������䣬��˵��m+1=3����m=2��A����ȷ��ͬһ��ѧ��Ӧ��ƽ�ⳣ��ֻ���¶��йأ�����ƽ���¶Ȳ��䣬������ƽ���ƽ�ⳣ����ͬ��B����ȷ��m=2������ʼ��X��Y֮��Ϊ1:2����Ӧ�������ɷ���ʽ��֪��Ӧ��X��Y֮��Ϊ1:2����X��Y��ƽ��ת����֮��Ϊ1:1��C����ȷ��m=2����÷�ӦΪ��Ӧǰ��������������ķ�Ӧ���ʵڶ���ƽ��ʱZ�����ʵ���Ϊ��4��10%=0.4mol����Z��Ũ��Ϊ0.4mol��2L=0.2mol/L����D�������ѡD��

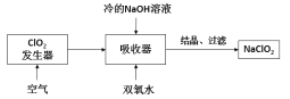
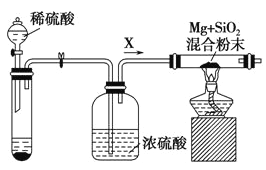
����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ����

�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ��������A������Ϊ___________��
��2���ü�װ����������MnO4- ����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽__________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ�
�ٴٽ�����������Ӧ��
��_______________________________��
��4�����ȱ����װ�ã����ܷ����ĸ���Ӧ�Ļ�ѧ����ʽΪ___________________����װ�õ�������_________________��
A.��ȥδ��Ӧ����������ֹ��Ⱦ����
B.��ֹ������CO2���������װ��
C.��ֹˮ����������װ�õ��Թ���ʹ����ˮ��
D.��ֹ������O2������װ�õ��Թ���ʹ��������
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2 ����_______________�����ţ���
A. H2O2��Һ B. FeCl3��Һ������KSCN�� C. AgNO3��Һ D. ��ˮ
��6����Ӧ����ȥ����1.19g����Ӧ������װ�õ��Թ����ռ���2.38gSnCl4����SnCl4�IJ���Ϊ________��������3λ��Ч���֣�
����Ŀ�������£�������������Һ��
�� | �� | �� | �� | �� |
0.1 mol��L-1 CH3COOH��Һ | 0.01mol��L-1 CH3COOH��Һ | pH��2 CH3COOH��Һ | 0.1 mol��L-1 NaOH��Һ | 0.1mol��L-1 ��ˮ |
�ش��������⣺
��1����Һ��ϡ�͵�ԭ����10�������ҺpH______����Һ��pH������������������������������ͬ�����ٺ͢�����Һ��ˮ�������c(H��)����_______��
��2������ͬ�¶�ʱ100mL �ڵ���Һ��10mL �ٵ���Һ��Ƚϣ�������ֵǰ�ߴ��ں��ߵ���_______
A �к�ʱ����NaOH���� B ����̶�
C ˮ�������c(H��) D CH3COOH�����ʵ���
��3����ˮϡ�͢�ʱ����Һ������ˮ�������Ӷ���С����______������ĸ����
A��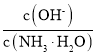 �������������� B��
�������������� B��
C��c��H+����c��OH-���ij˻���������D�� OH-�����ʵ���
��4��������N2H4������ˮ�Լ��ԣ���ԭ���백���ƣ�������Բ��簱ǿ��д��������ˮ�ʼ��Ե����ӷ���ʽ��________��