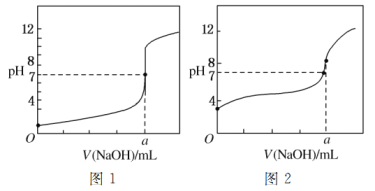
��Ŀ����
����Ŀ�������£���������Һ��pH(��֪lg2��0.3)��
��1��0.1mol��L��1��CH3COOH��Һ(��֪CH3COOH�ĵ��볣��Ka��1.8��10��5)��
��2��0.1mol��L��1NH3��H2O��Һ(NH3��H2O�ĵ����Ϊ����1%������ȣ�![]() ��100%)��
��100%)��
��3����pH��8��NaOH��pH��10��NaOH��Һ�������ϡ�
��4����pH��5��������pH��9��NaOH��Һ�������11��9��ϡ�
��1��pH��___����2��pH��___����3��pH��___����4��pH��___��
���𰸡�2.9 11 9.7 6
��������
��1��-��2���������ᡢ����ĵ���ƽ�ⳣ�����м��㣻
��3��-��4������c��OH-��(��)= ![]() ���㣻����c��H+��=
���㣻����c��H+��= ![]() ���м��㡣
���м��㡣
��1��CH3COOH�ĵ��볣��Ka��1.8��10��5����0.1mol��L��1��CH3COOH��Һ��������Ũ��Ϊxmol/L���� = 1.8��10��5��x
= 1.8��10��5��x![]() ��pH=2.9��
��pH=2.9��
��2�� OH-Ũ��Ϊ=0.1��1%=1��10��3�� ��PH=11��
��PH=11��
��3����pH��8��NaOH��pH��10��NaOH��Һ�������ϣ���Ϻ�OH-Ũ��Ϊ![]() ��
�� ��PH=9.7��
��PH=9.7��
��4����pH��5��������pH��9��NaOH��Һ�������11��9��ϣ����������Ϻ�H+Ũ��Ϊ![]() ������PH=6��
������PH=6��