��Ŀ����
�±���Ԫ�����ڱ���һ���֣�����Ҫ��ش��������⡣
��1��ʮ��Ԫ���л�ѧ��������õ�Ԫ����________����Ԫ�ط��ţ���
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����________���ѧʽ����
��3��IԪ�ظ�AԪ���γɻ�����ĵ���ʽ��________���������ոû�����ʱ�������________ɫ��
��4��G�ĵ��ʺ�B������������Ӧˮ���ﷴӦ�����ӷ���ʽ��__________________��
Ԫ��A��F���γ����ֻ����д�����н��ȶ��Ļ�������CO2��Ӧ���������Ļ�ѧ����ʽ ________________________��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 2 | | | | E | H | F | I | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | | |
��1��ʮ��Ԫ���л�ѧ��������õ�Ԫ����________����Ԫ�ط��ţ���
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����________���ѧʽ����
��3��IԪ�ظ�AԪ���γɻ�����ĵ���ʽ��________���������ոû�����ʱ�������________ɫ��
��4��G�ĵ��ʺ�B������������Ӧˮ���ﷴӦ�����ӷ���ʽ��__________________��
Ԫ��A��F���γ����ֻ����д�����н��ȶ��Ļ�������CO2��Ӧ���������Ļ�ѧ����ʽ ________________________��
��1��Ar ��2��NaOH ��3��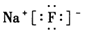 ��
��
��4��Cl2+2OH- = Cl- + ClO- + H2O
(5)2Na2O2+2CO2=2Na2CO3+O2��
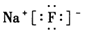 ��
����4��Cl2+2OH- = Cl- + ClO- + H2O
(5)2Na2O2+2CO2=2Na2CO3+O2��
�����������Ԫ�����ڱ���֪��A:Na B:K C:Mg D: Al E:C F:O G:Cl H:N I:F R:Ar
��1��ArΪ�������壬�����
��2��A ��C ��D����Ԫ�طֱ��Ӧ��ˮ����Ϊ NaOH�� Mg(OH)2��Al(OH)3����NaOH������ǿ��
��3��
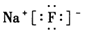 ������Ϊ���ӻ������Ԫ����ɫ��ӦΪ��ɫ��
������Ϊ���ӻ������Ԫ����ɫ��ӦΪ��ɫ����4��B������������Ӧ��ˮ����Ϊǿ���ȫ���롣�ʷ�Ӧ����ʽ ΪCl2+2OH- = Cl- + ClO- + H2O
��5��A��F�γ�Na2O��Na2O2�������ʣ����к��߸��ȶ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
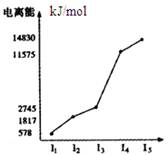
 ���˴Ź��������ں�̼������Ľṹ�������й�
���˴Ź��������ں�̼������Ľṹ�������й� ��˵����ȷ���ǣ� ��
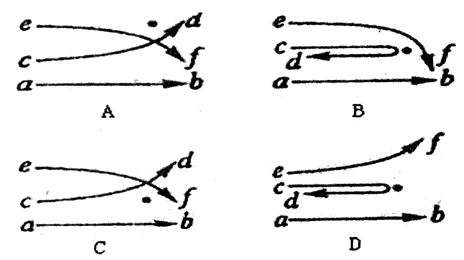
��˵����ȷ���ǣ� �� ���Ӻ�������Ľ�Ϊ�˽���ʵ�����������ԭ�ӵĺ�ʽ�ṹѧ˵������ͼ�У��ڵ��ʾ��ԭ�Ӻ˵�λ�ã�����ab��cd��ef��ʾ������ԭ�Ӻ˸�����
���Ӻ�������Ľ�Ϊ�˽���ʵ�����������ԭ�ӵĺ�ʽ�ṹѧ˵������ͼ�У��ڵ��ʾ��ԭ�Ӻ˵�λ�ã�����ab��cd��ef��ʾ������ԭ�Ӻ˸�����