��Ŀ����
����Ŀ��
������A�Ƿ���ʽΪC7H8O2����Ԫ��״�������˴Ź�������������壬�ҷ����֮��Ϊ3:1:2:2,����ͼ��ʾ��ת����

��֪:�ٺ��ʻ��Ļ��������������Լ�������Ӧ ��
��
�������ǻ�����ͬһ��̼ԭ���ϼ����ȶ�������ˮ: 
����������Ϣ���Իش��������⣺
(1)д��������A�к��������ŵ�����___________________��
(2)д��������B�Ľṹ��ʽ��___________________��
(3)A��B�ķ�Ӧ������______________________
(4)��д��D��CH4O��Ӧ�Ļ�ѧ����ʽ��__________________��
��д��F��G��Ӧ�Ļ�ѧ����ʽ��____________________��
(5)A�����ڷ����廯�����ͬ���칹����______�֣�д����������������A��һ��ͬ���칹��W�Ľṹ��ʽ��________________(�����������칹>��
�ٺ�������ȡ����
�ڱ����ϵ�һ�ȴ���ֻ������
��1 mol W�������������Ʒ�Ӧ����1 mol H2
���𰸡� ���� ![]() �ӳɷ�Ӧ����ԭ��Ӧ����
�ӳɷ�Ӧ����ԭ��Ӧ����  H2O
H2O  13
13 
����������A��B����ʽ��֪��A�����������ӳɷ�Ӧ����B��B����������ˮ������C��״�����A��Ӧ������������Ϸ���ʽ��֪A������2��̼̼˫��������AΪ��Ԫ��״��������ֲ�ͬ����ĸ���֮��Ϊ3��1��2��2�����G(![]() )��Ԫ���Ǽܿ��Ƶ�A�Ľṹ��ʽΪ��
)��Ԫ���Ǽܿ��Ƶ�A�Ľṹ��ʽΪ��![]() ��A��H2�����ӳɷ�Ӧ����̼̼˫��������BΪ
��A��H2�����ӳɷ�Ӧ����̼̼˫��������BΪ![]() ��B����������ˮ��õ��״���C(
��B����������ˮ��õ��״���C(![]() )��C���ữ�õ�DΪ
)��C���ữ�õ�DΪ![]() ��������Ϣ�ٺ͢ڿ�֪Dת��ΪEΪ
��������Ϣ�ٺ͢ڿ�֪Dת��ΪEΪ![]() ���ٸ�����֪�ٺ͢���E��ת��FΪ
���ٸ�����֪�ٺ͢���E��ת��FΪ![]() ��
��
(1)������������֪��A�Ľṹ��ʽΪ��![]() �����京��������Ϊ���������ʴ�Ϊ��������
�����京��������Ϊ���������ʴ�Ϊ��������
(2)������������֪��B�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)A��B�Ǽӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
(4)��D(![]() )��״�����������Ӧ������(
)��״�����������Ӧ������(![]() )����Ӧ����ʽΪ��
)����Ӧ����ʽΪ��![]() ����F��G�ķ�Ӧ�Ļ�ѧ����ʽΪ��
����F��G�ķ�Ӧ�Ļ�ѧ����ʽΪ��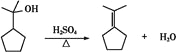 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
�� ��
��
(5) AΪ��![]() ��A�����ڷ����廯�����ͬ���칹����
��A�����ڷ����廯�����ͬ���칹���� (����3��)�������Ϻ���2���ǻ���1��������6��(2���ǻ�λ����λ����2�֡�2���ǻ�λ�ڼ�λ����3����2���ǻ�λ�ڶ�λ����1��)��
(����3��)�������Ϻ���2���ǻ���1��������6��(2���ǻ�λ����λ����2�֡�2���ǻ�λ�ڼ�λ����3����2���ǻ�λ�ڶ�λ����1��)��![]() (����3��)��
(����3��)��![]() ����13�֣��ٺ�������ȡ�������ڱ����ϵ�һ�ȴ���ֻ����������1 mol W�������������Ʒ�Ӧ����1 mol H2������������A��һ��ͬ���칹��W�Ľṹ��ʽΪ
����13�֣��ٺ�������ȡ�������ڱ����ϵ�һ�ȴ���ֻ����������1 mol W�������������Ʒ�Ӧ����1 mol H2������������A��һ��ͬ���칹��W�Ľṹ��ʽΪ ���ʴ�Ϊ��13��
���ʴ�Ϊ��13�� ��
��

 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�����Ŀ���ⶨ���������Ȼ���С�մ�(��Ҫ�ɷ�ΪNaHCO3)�ĺ����ж��ַ�����
��1���������������ȷ���Ʒ���û�ѧ����ʽ��ʾ�ķ�Ӧԭ���ǣ�____________________��
II.��������ͨ�����___________��������Ӷ�ͨ���������С�մ�ĺ�����
��2��III.�ζ������ݻ�������Ӧԭ����NaHCO3+HCl��NaCl+H2O+CO2��,
NaHCO3�������ԣ��ʲ����÷�̪��ָʾ����������__________��ָʾ�����ñ�����ζ���Ʒ���ζ��յ�ʱ��������________________________
�ζ���ʵ�鲽������ͼʾ

��3�����������ܽ⡢���ݡ�����������Ҫ��������Ҫ�У�������ƽ��ҩ�ס�100mL������ƿ��___________��____________��___________��
��4����ȡ��20mL��ʹ�õĵζ�����ˮϴ��û���������ϴ���Եζ������Ӱ����_______����ƫ�ߡ���ƫ�͡�����Ӱ�족�������롰������ʵ�顱������__________________.
ij��ѧ��ȤС���ͬѧ���������ⶨijƷ��С�մ���NaHCO3����������������������ʵ�飺������Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
���� | ��Ʒ | ���ĵ�ϡ���� | ��Ӧ�����Һ |
����(g) | 4.5 g | 37.7 g | 40 g |
�Լ��㣺
��5�����ɵ�����CO2_________;��Ʒ�е�NaHCO3����������_______________(����������һλС��)





