��Ŀ����
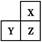
��֪X��Y��Z��W��R����Ԫ��,ԭ��������������,��ԭ��������С��20,XԪ�ص�ԭ��������Ԫ�ص�ԭ���а뾶��С��,Y��Wͬ����,Z��Wͬ����,YԪ��ԭ�ӵ������������Ǵ�����3��,Z��R�ֱ���ͬ�����н�������ǿ��Ԫ�ء�����˵������ȷ���ǣ� ��
| A���е�:X2Y��X2W |
| B����X��Y��Z��W����Ԫ����ɵĻ�����Ⱥ��й��ۼ��ֺ����Ӽ� |
| C��ԭ�Ӱ뾶:X��Y��Z��W��R |
| D��Y��W�γɵĻ�����WY2���γ��������Ҫ����֮һ |
C
����XԪ�ص�ԭ��������Ԫ�ص�ԭ���а뾶��С��,��XΪH;����YԪ��ԭ�ӵ������������Ǵ�����3��,��YΪO,Y��Wͬ����,��WΪS,Z��Wͬ������Z��R�ֱ���ͬ�����н�������ǿ��Ԫ��,��ZΪNa,RΪK��X2Y��ΪH2O,X2W��ΪH2S,�е�ǰ�߸�,A����ȷ;X��Y��Z��W��ɵĻ�����ΪNaHSO4(��NaHSO3),�Ⱥ������Ӽ��ֺ����ۼ�,B����ȷ;ԭ�Ӱ뾶:X(H)��Y(O)��W(S)��Z(Na)��R(K),C�����;WY2��SO2,���γ��������Ҫ����֮һ,D����ȷ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
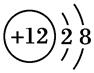 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��