��Ŀ����
����Ŀ������β������Ҫ�ɷ���CO��SO2��NO��NO2�ȡ�
��1�����ð�ˮ���Խ�SO2�������������գ�ԭ������ͼ��ʾ��
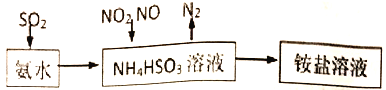
��25��ʱ����pH=5�� NH4HSO3��Һ�У�c(SO32��)+c(NH3��H2O)��c(H2SO3)=__________mol/L(��ȷֵ)
����д��NO2��NO�������1��1������ʱ��Ӧ�����ӷ���ʽ_____________________��
��2�����й�����Ŀǰ���ڳ����Զ�������(TiO2)���ֽ�����β�����о���
����֪��2NO(g)+O2(g)=2NO2(g) ��H1=��113.0kJ��mol��1
3NO2(g)+H2O(g)=2HNO3(g)+NO(g) ��H2=��138.0 kJ��mol��1
TiO2��β������ԭ���ɱ�ʾΪ��2COg)+O2(g)![]() 2CO2(g) ��H3
2CO2(g) ��H3
��2H2O(g)+4NO(g)+3O2(g)![]() 4HNO3(g) ��H4=______________________��
4HNO3(g) ��H4=______________________��
����O2��H2O(g)Ũ��һ����������ģ��CO��NO�Ľ������õ��併����(��ת����)��ͼ1��ʾ�������T1��NO�������½��Ŀ���ԭ��______________________��
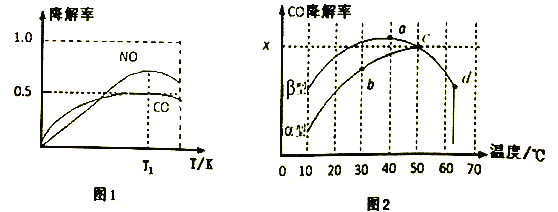
��3�����������Ҳ�ɽ���CO����ͼ2Ϊ�ڲ�ͬ������϶�����������(��������)�ڲ�ͬ�¶��£���Ӧ��ͬʱ�䣬���CO�����ʱ仯�������ͼ�ش��������⣺
����֪��50��ʱ��������������������У�ƽ��ʱO2Ũ��Ϊ0.01mol��L��1������¶���CO���ⷴӦ��ƽ�ⳣ��____________________________________________��(�ú�x�Ĵ���ʽ��ʾ)
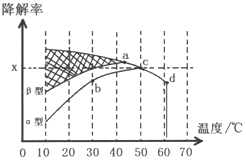
�ڿ����Ŷ��������������������Ϊ���壬��TiO2��Ϊ��Ч�����õ�TiO2���ܣ���10��~60����Χ�ڽ���ʵ�飬������ͼ�����߶�����![]() ����Ӱ����CO���������¶ȱ仯�����߿��ܳ��ֵ��������Χ(��ͼ�л���)��____________
����Ӱ����CO���������¶ȱ仯�����߿��ܳ��ֵ��������Χ(��ͼ�л���)��____________
��4��TiO2���ܵ��Ʊ�����������ˮ��Һ���Խ�����Ϊ�������е�⣬д�������ĵ缫��Ӧʽ____________________________________________��
���𰸡�10-5-10-9 NO+NO2+3HSO3-=N2+3SO42-+3H+ -615kJ/mol �¶����ߣ�ƽ�������ƶ� ![]()
(ͼ��˵������Ӱ����a��֮ǰ��![]() ���Ϸ���a��֮���غ�) Ti-4e-+2H2O=TiO2+4H+
���Ϸ���a��֮���غ�) Ti-4e-+2H2O=TiO2+4H+
��������
(1) ��25��ʱ����pH=5�� NH4HSO3��Һ�д��ڵ���غ㣺c(OH-)+ c(HSO3��)+2c(SO32��) =c(NH4+)+c(H+)�������غ㣺c(NH4+)+ c(NH3��H2O)= c(HSO3��)+c(SO32��)+ c(H2SO3)���ݴ˷�����
�ڸ�������ͼ֪��NO2��NO��NH4HSO3��Ӧ��+4�۵ĵ�����+4�۵�����NO2��NO��NH4HSO3��������N2��NO2��NO�õ��ӱ���ԭ����HSO3������������SO42�����ݴ���д���ӷ���ʽ��
(2)�ٸ�����֪����ʽ����Ŀ�귽��ʽ�����ݸ�˹���ɼ��㷴Ӧ����
�ڸ���ƽ���ƶ�ԭ���������������¶���ƽ�������ƶ���
(3)�ٻ�ѧƽ�ⳣ��Ϊ������Ũ��ϵ�����ݵij˻��뷴Ӧ��Ũ��ϵ�����ݳ˻��ı�ֵ��
�ھݴ����ܼӿ컯ѧ��Ӧ����������Ӱ��ƽ���ƶ���ͼ��
(4)������ʧȥ���ӷ���������Ӧ��д�缫��Ӧ����ʽ��
(1) 25��ʱ����pH=5�� NH4HSO3��Һ�д��ڵ���غ㣺c(OH-)+ c(HSO3��)+2c(SO32��) =c(NH4+)+c(H+)�������غ㣺c(NH4+)+ c(NH3��H2O)= c(HSO3��)+c(SO32��)+ c(H2SO3)����c(SO32��)+c(NH3��H2O)��c(H2SO3)= c(H+)- c(OH-)=��10-5-10-9��mol/L��
�ڸ�������ͼ֪��NO2��NO��NH4HSO3��Ӧ��+4�۵ĵ�����+4�۵�����NO2��NO��NH4HSO3��������N2��NO2��NO�õ��ӱ���ԭ����HSO3������������SO42������Ӧ�ķ���ʽΪ��NO+NO2+3NH4HSO3=N2+3NH4HSO4�����ӷ���ʽΪNO+NO2+3HSO3-=N2+3SO42-+3H+��
��ˣ�������ȷ������NO+NO2+3HSO3-=N2+3SO42-+3H+��
(2)�ٷ�Ӧ��2NO(g)+O2(g)=2NO2(g) ��H1=��113.0kJ��mol��1
��Ӧ����3NO2(g)+H2O(g)=2HNO3(g)+NO(g) ��H2=��138.0 kJ��mol��1
���ݸ�˹����(���3+���2)�÷�Ӧ2H2O(g)+4NO(g)+3O2(g)![]() 4HNO3(g)
4HNO3(g)
����H4=[(��113.0kJ��mol��1) ��3+(��138.0 kJ��mol��1) ��2]= -615kJ/mol
��ˣ�������ȷ������-615kJ/mol��
�������ϼ����֪��2H2O(g)+4NO(g)+3O2(g)![]() 4HNO3(g)Ϊ���ȷ�Ӧ�����ŷ�Ӧ�Ľ��У��¶�������ƽ�������ƶ������HNO3Ũ�Ƚ�����
4HNO3(g)Ϊ���ȷ�Ӧ�����ŷ�Ӧ�Ľ��У��¶�������ƽ�������ƶ������HNO3Ũ�Ƚ�����
��ˣ�������ȷ���ǣ��¶����ߣ�ƽ�������ƶ���
(3)��CO�Ľ�����Ϊ
2CO(g)+O2(g)![]() 2CO2(g)
2CO2(g)
��ʼ(mol/L) a 0
�仯(mol/L)ax ![]() ax ax
ax ax
ƽ��(mol/L)a-ax0.01 ax
K=![]() =
=![]() =
=![]()
��ˣ�������ȷ������![]() ��
��
�ھݴ����ܼӿ컯ѧ��Ӧ����������Ӱ��ƽ���ƶ�����ͼΪ
 ��
��
��ˣ�������ȷ������ ��
��
(4)����ʧȥ���ӷ���������Ӧ���ʵ缫��Ӧ����ʽΪTi-4e-+2H2O=TiO2+4H+��
��ˣ�������ȷ������Ti-4e-+2H2O=TiO2+4H+��